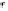Biomedical Engineering Reference
In-Depth Information
Net electron transfer
Fe(CN)
3
−
6
Fe(CN)
3
6
P
P
h
P*
Fe(CN)
4
−
6
P
+
P
+
Fe(CN)
4
−
6
Fe(CN)
3
6
P
C
p
R
p
R
e
/2
R
e
/2
E
p
(
t
)
R
s
I
(
t
)
I
(
t
)
C
m
Electrode
Electrode
R
m
Aqueous phase
(oxidant side)
Aqueous phase
(reductant side)
Membrane phase
FIGURE 15.1
A simple light-driven electron pump and the equivalent circuit. The bilayer lipid membrane (BLM) contains a
lipid-soluble magnesium porphyrin and separates two aqueous phases with asymmetric concentrations of potas-
sium ferricyanide (electron acceptor) and potassium ferrocyanide (electron donor). The symbol
P
stands for the
ground-state pigment, P
*
for the excited state pigment, and P
for the corresponding monocation. Interfacial
electron-transfer reactions between the membrane-bound pigment and the aqueous-borne electron donor/accep-
tor are coupled by diffusion of P and P
. Electrons are preferentially transported from the reductant side (where
the donor concentration is much greater than the acceptor concentration) to the oxidant side (where the acceptor
concentration is much greater than the donor concentration). The reverse reaction at the oxidant interface is also
shown. The equivalent circuit is shown at the bottom of the diagram. The photoelectromotive force (photoemf)
E
p
(
t
) with its internal resistance,
R
p
, is connected in series with the chemical capacitance,
C
p
, and the transmem-
brane DC resistance,
R
s
. The ordinary membrane (ionic) resistance,
R
m
, and capacitance,
C
m
, are placed in paral-
lel with the photoemf. The access impedance (resistance),
R
e
, includes the amplifier input impedance, the
electrode impedance, and the impedance of the intervening electrolyte solution. See text for further explanation.
(From Hong, F. T., Mauzerall, D. (1974). Interfacial photoreactions and chemical capacitance in lipid bilayers.
Proc. Natl. Acad. Sci. USA
71:1564-1568.)
the reductant side, and, conversely, causes less neutral P to appear at the oxidant side than
at the reductant side. As a consequence, the net transmembrane diffusion of P
generates
an electric current that flows from the oxidant side to the reductant side. Net diffusion of
the neutral ground-state P in the opposite direction is electrically silent. Thus, in the mem-
brane phase, the pigment molecules undergo cyclic oxidation and reduction—oxidation at
the oxidant side and reduction at the reductant side. The “counter-current” transmem-
brane flows of P and P
thus give rise to a sustained DC photoelectric current (from the
oxidant side to the reductant side). The two interfacial redox reactions are coupled by dif-
fusion of P and P
in opposite directions (coupled interfacial electron-transfer reactions).
The hydrophobic porphyrin molecules thus serve as the electron shuttles.
Under illumination with continuous light, a steady-state (sustained) photocurrent or
photovoltage is observed; the polarity is consistent with electron movement from the