Biomedical Engineering Reference
In-Depth Information
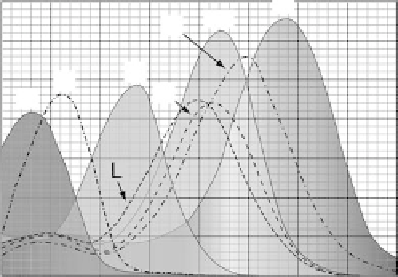
arrangement of the protein in the membrane imparts tremendous stability. Figure 14.5 illus-
trates the absorption spectra of the various BR intermediates, several of which are impor-
tant to the protein's role in biomolecular electronics. Perhaps the most versatile aspects of
BR's utility in device applications are the ability to interrogate the protein by multiple
means, by examining modulation of either the optical or photovoltaic properties as a result
of external stimuli, and the researchers' ability to improve BR's performance through chem-
ical and genetic means.
To integrate BR, or any protein for that matter, into an environment designed for suc-
cessful signal transduction, several conditions must be met. These include (1) maintaining
the protein in a state that is functional and stable, (2) proper orientation of the protein, (3)
ensuring that there is adequate coupling between the protein and the environment for effi-
cient signal input and output, (4) proper encapsulation of the protein to hold it in place and
protect it from degradation (natural or otherwise), and conversely (5) protecting the syn-
thetic environment from any adverse affects due to the protein. For the specific case of BR,
some of these issues are less important than others. Because of its native environment (the
purple membrane, or PM), BR is already more rugged and stable than most proteins, and
is naturally protected from oxidation and (to a large extent) microbial degradation. Loss of
function due to dehydration is more of a problem. But the purple membrane has its own
set of challenges, specifically due to its size (~0.5-1
m). This size is the same order of mag-
nitude as the visible wavelength used to excite or interrogate the protein (500-1,000 nm),
and therefore will induce light scattering. Furthermore, when faced with the challenge of
fabricating microelectronic devices, the average purple membrane patch size is often larger
than the average lithographic feature size, making precise deposition of the protein diffi-
cult. New methodologies may have to be developed to overcome these problems. At least
one methodology for patterning PM fragments has been reported in the literature;
Brizzolara et al. [77-79] genetically modified BR to contain a cysteine (an amino acid con-
taining a sulfhydral group) at the membrane surface. The sulfhydryl group can ligate to a
gold surface that has been lithographically patterned previously. PM patches adhere only
to the surfaces patterned with gold. However, this technique was only marginally effective,
in that coverage was not complete and sulfhydryl contaminants polluted the gold surface.
Large patch size may have been part of the problem, and it has been demonstrated that
sonication can be used to reduce patch size (unpublished in-house data). Regardless, this
ligation technique is not likely to produce a uniform PM coating on semiconductor
surfaces. Other methods of depositing PM monolayers or films involve hydrophobic
and hydrophilic bonding, such that the protein only bonds to hydrophilic surfaces [80].
One method relies on depositing PM over a previously patterned silicon surface that is of
µ
O
bR
K
6
P
5
M
N
Q
4
3
FIGURE 14.5
(See color insert)
Absorption spectra of the
various bacteriorhodopsin intermediates are
shown. The bR, M, and O states are of partic-
ular interest to most of the applications
developed around bacteriorhodopsin's
unique photochromokinetic and photovoltaic
properties.
2
1
350
400
450
500
550
600
650
700
750
Wavelength (nm)