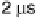Biomedical Engineering Reference
In-Depth Information
of phycobiliproteins for two-photon fluorescence microscopy of three-dimensional (3-D)
biological samples as well as potentially in 3-D optical memories.
Photovoltaic properties of phycobiliproteins have also been studied. We investigated
charge transport phenomena by analyzing the dark current-voltage and photocurrent char-
acteristics obtained across gold-phycobiliprotein-gold samples (23). A photovoltaic effect
was observed for the gold-phycoerythrin-gold sample. At low intensity levels, the photo-
current closely followed Onsager's law of geminate recombination in three dimensions.
1.2.1.1.2 Bacteriorhodopsin
Bacteriorhodopsin (bR) is the light-transducing integral membrane-bound protein found in
the purple membrane of
Halobacterium halobium
. The single subunit protein is 248 amino
acids in length and contains a retinylidene chromophore covalently linked via Schiff's base
linkage to the lysine-216 amino acid residue (24). In the bacterium, bR acts as a light-driven
proton pump. Upon absorption of a photon, bR undergoes a photocycle characterized by a
series of well-defined intermediates with different absorption spectra (25). In Figure 1.10, we
present a schematic of the photocycle. The defined optical states are characterized by the
changes in absorption maximum indicated by the states' subscript wavelengths. The solid
arrows show the thermal decay pathways and decay times. Dashed lines correspond to pho-
ton-driven processes with some quantum yields indicated. When oriented, bR films possess
very interesting optoelectronic properties that have the potential to be used in a number of
different application areas including real-time holography, artificial retinas, optical neural
networks, and image processing (26-32). Like all proteins, bR has the advantage that genetic
variants with potentially improved properties for a particular application can be screened to
select for a desired alteration. Since the study of this protein was not a major focus of our
Center, we do not discuss its interesting properties comprehensively in any further detail,
but focus only on our use of it in specific applications.
Aside from its interesting photocycle, one of the very attractive features of this integral
membrane protein is its exceptional stability. By the standards of protein chemistry, the
bR protein and its proton pumping photocycle are extremely robust to denaturation or
normal oxidative damage. A second attractive feature is its ability to be ordered. The bR
protein occurs in a regular hexagonal lattice within the bacterial membrane and is
uniquely oriented to the two membranes faces. The membrane fragments found in a
typical bR preparation can be easily oriented using electric fields (33), a property that
results from the membrane possessing differential charge characteristics upon its intracel-
lular and extracellular faces. Taking advantage of these features, we investigated the bR
70%
bR
570
0.5 ps
5 ms
FIGURE 1.10
Photochemical cycle of the bacteriorhodopsin (bR) molecule.
In this schematic summary, dashed arrows indicate photon-
driven processes. The solid arrows show the thermal decay
path. Also indicated are the lifetimes of the intermediate
states, as well as the quantum yields for the forward and
backward photoreactions of the bR, J, K, and M states.
Reprinted from Chen, Z., Lewis, A., Kumar, J., Tripathy, S.K.,
Marx, K.A., Akkara, J., Kaplan, D.L. (1994). Second
Harmonic Generation of Bacteriorhodopsin and Its
Application for Three-Dimensional Optical Memory. In: M.
Alper, H. Bayley, D. Kaplan, M. Navia, eds. Biomolecular
Materials by Design,
Proc. Mat. Res. Soc.
, 330:263-268. With
permission from the Materials Research Society.
J
625
O
660
3 ps
70%
K
610
3 ms
70%
N
520
H
+
L
550
1 ms
M
412
H
+