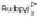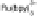Biomedical Engineering Reference
In-Depth Information
protocol for detecting DNA hybridization and even for DNA mismatch detection based
on attaching thiolated DNA probes along the gold NP-CNTs hybrid has been reported
recently, and such sensor systems can be extended for RNA and peptide nucleic acid
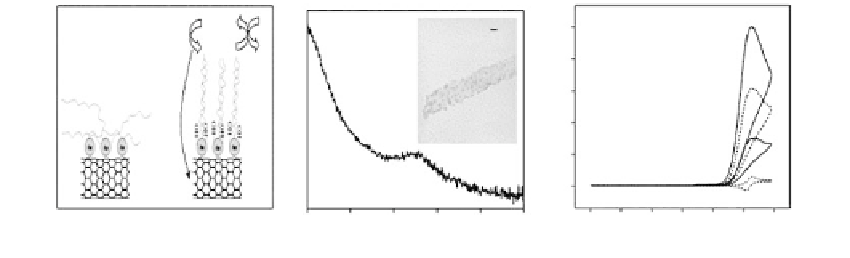
(PNA) analysis [156]. This protocol (Figure 12.18) relies on the attached gold NPs with
CNTs as an anchor to capture single-strand DNA probes. CNTs act as a support substrate
that serves as a fast electron-transfer center and captures the catalytic oxidation current
of guanine bases. In this system, a redox mediator such as Ru(bpy)
3
2+
,
which amplifies
guanine oxidation based on an electrochemical mechanism, has often be adopted to gen-
erate larger electrochemical signals.
12.5.1.1.4 Carbon Nanotube-Based Nanoelectrode Arrays
The performance of electrodes with respect to response speed and sensitivity is known to
scale inversely with the electrode size. It is of interest for biosensing to reduce the size of
electrodes close to the size of biomolecules. Unlike employing randomly deposited CNT
films as a biosensor platform, the latest advances in the fabrication of well-controlled
aligned CNT arrays have attracted attention for developing ultrasensitive electrochemical
biosensors [79,157-161]. Many approaches to prepare CNT arrays have been described by
various workers. For example, high-temperature catalytic decomposition of hydrocarbon
precursors on metal-modified substrates has yielded multiwall nanotube-based aligned
array geometries [24,27].
Analogous to covalent functionalization of CNTs, carboxyl groups generated by oxida-
tive scission of CNTs have exhibited considerable affinity for perpendicular alignment of
CNTs [162,163]. For example, a dense array of DMF oxidized SWNTs has been fabricated
using a metal-assisted self-assembly method via covalent bonds between the end-car-
boxy group of CNTs and hydroxyl group of the surface-immobilized Fe
3+
layer [164].
Similarly, cystamine monolayer-functionalized gold substrate can also provide an excel-
lent platform to covalently attach carboxylic acid-functionalized SWNTs for vertical
alignment of CNTs. The carboxylic groups at the free ends of the standing CNTs can fur-
ther form covalent bonds selectively to the biomolecular probes (such as ferrocene [158],
DNA [165,166], and protein for biosensor applications [79,167]) (Figure 12.19). Although
the structural alignment of the forest-like CNT arrays allows the direct contact of redox
(a)
SH-(-)-ssDNA probes
(b)
15
10 nm
12
9
(d)
(c)
6
(b)
3
(a)
0
300
400
500
600
800
800
0.0 0.2 0.4 0.6 0.8 1.0 1.2
Wavelength (nm)
Potential (V)
FIGURE 12.18
Left: (a) Schematic illustration of self-assembly of thiolated oligonucleotides onto Au-CNT hybrid. The use of
MCH assists the erection of ssDNA and facilitates hybridization of complementary oligonucleotides, which is
detected via mediator. Middle: (b) UV-Vis absorption spectrum of MWNT bound with gold nanoparticles. The
inset shows the TEM image of a MWNT coated with gold nanoparticles. Right: cyclic voltammograms (CV) of
Ru(bpy)
2
(30
M) in 50 mM phosphate buffer at pH 7 with 700 mM NaCl at 25 mV s
1
: when Au-CNT
-elec-
trode is modified with (a) MCH only; (b) complementary ss-oligonucleotide (2
); (c) two-mismatched ss-
oligonucleotide (3
); and (d) single-mismatched ss-oligonucleotide. (From Lim, S.-H., Wei, J., Lin, J. (2004).
Electrochemical Genosensing Properties of Gold Nanoparticle-Carbon Nanotube Hybrid.
Chem. Phys. Lett.,
400, 578-582.)