Biomedical Engineering Reference
In-Depth Information
1.00 E
−
08
(1) = Undoped
(
2) = Doped
(2)
5.00 E
−
09
(1)
0.00 E+00
−
100
−
50
0
50
100
(1)
−
5.00 E
−
09
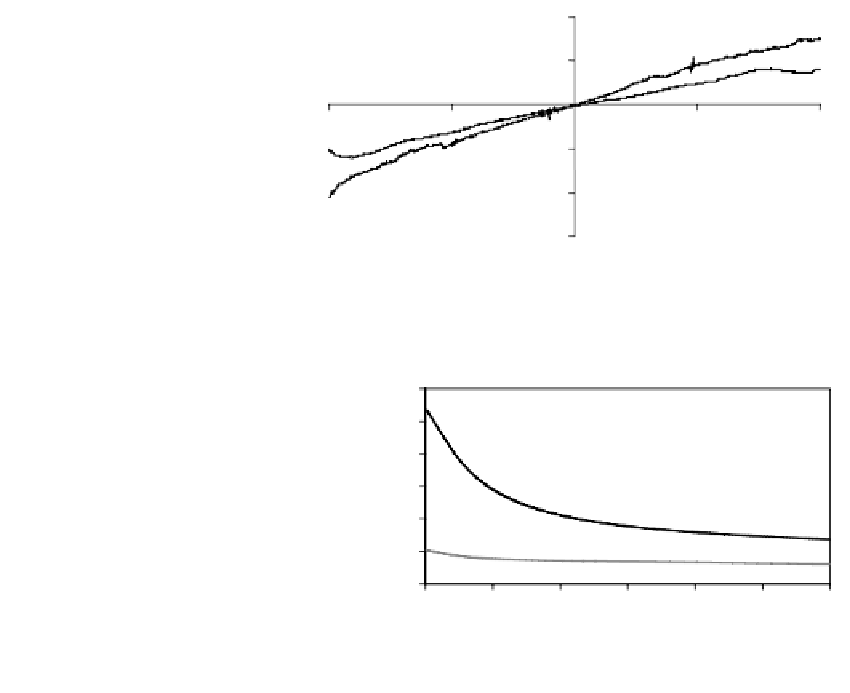
FIGURE 9.9
V
/
I
characteristics of PDMA-MWNTs
nanocomposite in both the undoped
(curve 1) and doped (curve 2) forms.
The material showed a quasi-linear
behavior.
Current (A)
−
1.00 E
−
08
(2)
1.50 E
08
−
−
Potential (V)
Variation of current vs. time after doping in confined
volume
6.00E
−
06
5.00E
06
1 = 0.5 mL
2 = 0.2 mL
−
1
4.00E
−
06
3.00E
−
06
2.00E
−
06
2
1.00E
−
06
FIGURE 9.10
Response of the sensor device to HCl
vapors.
0.00E+00
0
50
100
150
200
250
300
Time (s)
of PDMA-MWNTs nanocomposite in the undoped and doped forms evidenced by
curves 1 and 2, respectively, are illustrated. The instability of the doping process allowed
us to fabricate a spontaneous reversible sensor for acid vapors (7) by setting up a com-
parative potentiometric circuit and engineering the sensitive element directly on the
circuit board. To test the response of the fabricated device after exposing to HCl vapors,
an experimental set-up was realized as following. The device was placed inside a con-
tainer of 120 ml and sealed by means of a parafilm layer, letting the wires out through it
to be connected to the electrometer. Volumes of 0.2 and 0.5 ml of saturated vapors of HCl
were injected through the parafilm membrane. Current versus time measurements at a
fixed bias of 10 V were then carried out. The relative results obtained from the experi-
ments are shown in Figure 9.10. It is observed that the system tends to return to the ini-
tial conditions of resistance after about 3 min since the injection of HCl vapors. This
behavior is the consequence of the instability of EB/ES equilibrium, which can be shifted
to the stable formation of the ES form only by means of a continuous dynamic inflation
of acid vapors. Curve 1 represents the saturation level of the sensor device valuable in a
concentration of 4200 ppm of HCl vapors, since higher levels of concentration gave the
same results. Curve 2 shows the data obtained for an intermediate concentration between
the absence and the saturation of vapors, and the device evidenced a response in func-
tion of HCl vapors concentration. The experiment was carried out by quickly injecting
the acid vapors into the system containing the sensor device; and the acquisition of data
started immediately after the complete injection (7).
It will be the future goal of our research to study other nanocomposites synthesized by
using different monomers and concentrations of MWNTs inside the polymeric matrix for
a wide range of gas-sensor applications.