Biomedical Engineering Reference
In-Depth Information
has been made (58). The influence of environmental conditions such as pH, temperature,
and ionic strength on the equilibrium swelling ratio of physically cross-linked networks of
these gels was investigated. The effects of gelation cure time and initial polymer concen-
tration on the equilibrium swelling ratio and soluble fraction of the hydrogels were also
studied. It was found that the soluble fraction linearly correlated with the initial polymer
concentration at higher gelation times. Soluble fraction results suggest that final hydrogel
water content may be controlled by both initial polymer concentration and gelation time.
Differentially charged analogs of block copolymers containing repeating sequences from
silk (GAGAGS) and elastin (GVGVP) were synthesized using genetic engineering tech-
niques by replacing a valine residue with glutamic acid. The sensitivity to pH and tem-
perature was examined at various polymer concentrations, ionic strengths, and polymer
lengths. The polymers transitioned from soluble to precipitate state over narrow temper-
ature ranges. The transition temperature
T
-
t
(the temperature at which half-maximal spec-
trophotometric absorption was observed) increased with increasing pH up to pH 7.0 and
leveled off above this value for the Glu-containing polymer (17E)(1
t
).
T
-
t
was independ-
ent of p
I
for the Val-containing polymer (17V)(1
t
). It decreased with increasing ionic
strength, polymer concentration, and polymer length for both polymers. These results
suggest that by substituting charged amino acids for neutral amino acids at strategic loca-
tions in the polymer backbone and by control of the length of silk-elastin-like block
copolymers using genetic engineering techniques, it is possible to precisely control sensi-
tivity to pH, temperature, and ionic strength. Equilibrium swelling studies demonstrated
that these hydrogels are relatively insensitive to environmental changes such as pH, tem-
perature, and ionic strength. Over the concentration range studied, it was found that an
increase in gelation time at 37ºC resulted in lower hydrogel weight equilibrium swelling
ratios, which corresponds to less soluble polymer released postgelation. Together, these
results have implications for the controlled delivery of bioactive agents from silk-elastin-
like hydrogels.
Cai et al. (59) have applied a magneto-elastic transducer-based biosensor using a pH-
sensitive gel (Figure 7.5). The magneto-elastic transducer vibrates in a time-varying mag-
netic field at a resonance frequency the value of which inversely depends on sensor mass
loading. The glucose biosensor was made by coating with a pH-sensitive polymer and a
GOD layer on the transducer surface. The GOD-catalyzed oxidation of glucose produced
gluconic acid, causing shrinking of the pH-responsive polymer and decrease in mass. The
changes in magnetic flux were remotely detected using a pickup coil. The sensitivity of the
glucose biosensor was a function of ionic strength. The response was linear up to 10 mM,
and the detection limit was 0.6 mM.
A miniature conductimetric pH sensor based on the measurement of the conductivity of
a pH-responsive hydrogel has been described (60). The microfabricated sensor consisted
of electrode arrays coated with a photo-lithographically patterned hydrogel membrane.
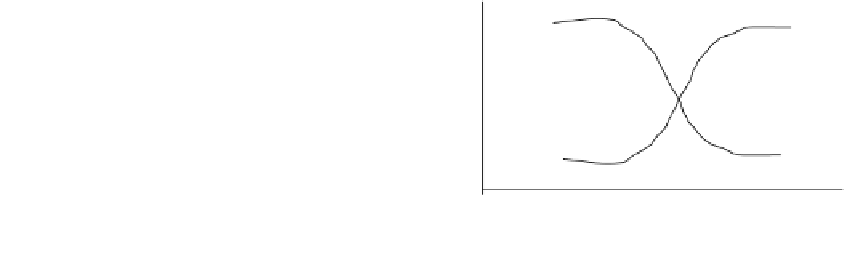
Basic
groups
Acidic
groups
3
7
12
FIGURE 7.5
Swelling behavior of pH-sensitive hydrogel materials.
pH