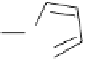Biomedical Engineering Reference
In-Depth Information
O
H
NH
(CH
2
)
4
(CH
2
)
3
N
O
FIGURE 4.9
Structure of biotinylated pyrrole.
S
O
obtained if the doping anion is irreversibly captured in the polymer matrix. The incorpo-
ration of anionic complexing ligands to the CPs comes under this category [48]. PPY func-
tionalized with biomolecules and other receptors such as 2,6-pyridinedicarboxylic acid
and ethylenediaminetetraacetic acid have been prepared using this method [49]. We also
have used this procedure to incorporate 2(2-pyridylazo)chromotropic acid anion (PACh
2
)
as a dopant within PPY and utilized it for the selective determination of lead in water
down to 10 ng/mL [50]. In this technique, an aqueous solution of pyrrole containing the
disodium salt of 2(2-pyridylazo)chromotropic acid was electropolymerized at constant
potential. Since PACh
2
was the only anion present in the solution, it was incorporated in
the growing cationic PPY film as the charge-balancing counter anion. The PACh
2
acted
both as an electrolyte and a dopant. Wang and Musameh [51] also recently demonstrated
a one-step preparation route of amperometric enzyme electrodes based on incorporating
a CNT dopant and glucose oxidase (GO
x
) enzyme within an electropolymerized PPY film
[51]. The CNT dopant retained its electrocatalytic activity to impart high sensitivity upon
entrapment within the PPY network. Such simultaneous incorporation of CNT and GO
x
thus imparts biocatalytic and electrocatalytic properties onto amperometric transducers
and represents a simple and effective route for preparing enzyme electrodes.
The third procedure is the postpolymerization functionalization method. In this case, the
functionalization is performed after the polymerization. An appropriate functional group in
a polymer is allowed to covalently bind to another functional group of a specific molecule.
This approach requires the electrosynthesis of CPs possessing reactive entities used as
anchoring points to graft the functional groups. A good example is the recent postpolymer-
ization functionalization of poly(N-subsituted pyrrole) film [52]. The
-ferrocene ethylamine
used as redox probe was immobilized via a chemical coupling on the surface of a preformed
PPY film bearing activated ester groups on to which biotin entities were immobilized.
The fourth way involves entrapment of target molecules within CPs. This route involves
the application of an appropriate potential to the working electrode soaked in a solution con-
taining the target molecule, the monomer, and dopant. It allows spatially controlled deposi-
tion of the molecules in CP matrices [53-56]. Moreover, the entrapment of the molecules
occurs without any chemical reaction that could affect their activity. Electrochemical poly-
merization ensures that films are prepared in a rapid one-step procedure. Further, this
method enables the exact control of the thickness of the polymer based on the measurement
of the electrical charge passed during the electrochemical polymerization. However, in some
cases, physical entrapment in polymer films and steric hindrances may drastically reduce the
catalytic activity and flexibility of some immobilized biomolecules such as enzymes [57,58].
4.3.1
Functionalization of Conducting Polymer Nanowires
The functionalization of CP NWs has recently generated a lot of interest due to the fact
that polymeric nanowires are characterized with higher conductivity than polymeric
macroscopic films, and their electronic conductance is strongly influenced by minor