Biomedical Engineering Reference
In-Depth Information
in the overlap integral of the fluorescence emission wavelength for the donor. Alternatively,
the acceptor can be a “dark absorber” which does not emit fluorescence radiation such that
the only signal that is observed is from the donor when it moves sufficiently far enough
away from the acceptor species. There are several solution-based assays that operate upon
this principle, which have been developed in recent years, and “Molecular Beacons” are one
such example, which have been used for single molecule detection.
Molecular beacons (MB) are single-stranded probe oligonucleotides that possess a donor
and an acceptor bound to the terminal ends of the sequence. A specified number of bases
at the 3' and 5' ends of the sequence (usually 4-7 bases) are complementary to one another
allowing the MB to adopt a hairpin or “stem-loop” conformation. This conformation allows
the donor and acceptor to be in close proximity to one another and quenching of the donor
species by the acceptor species results. The MB maintains this conformation until a target
sequence of sufficient complementarity to the MB is introduced which allows the system to
overcome the energetics of binding of the hairpin structure, which is then dissociated, and
binds to its complementary sequence. This binding event elongates the MB sequence which
increases the distance between the donor and acceptor species beyond the Förster distance
and the fluorescence of the donor species is restored. Several combinations of donor/accep-
tor pairs can be considered including two fluorophores, a fluorophore and a quencher that
does not emit fluorescence radiation, a fluorophore and an intercalator, a fluorophore and
a gold particle, and a fluorophore and quantum dot (Figure 2.3). Quantum dots are small
semiconductor particles of approximately 10 nm dimension, which have very large quan-
tum yield and very narrow emission spectra. The narrow spectral emission band and the
lack of any significant photobleaching suggest that quantum dots can be excellent labels for
300
800
300
800
Excitation
wavelength (nm)
Excitation
wavelength (nm)
10 nm
300
800
300
800
Emission
wavelength (nm)
Emission
wavelength (nm)
(A)
(B)
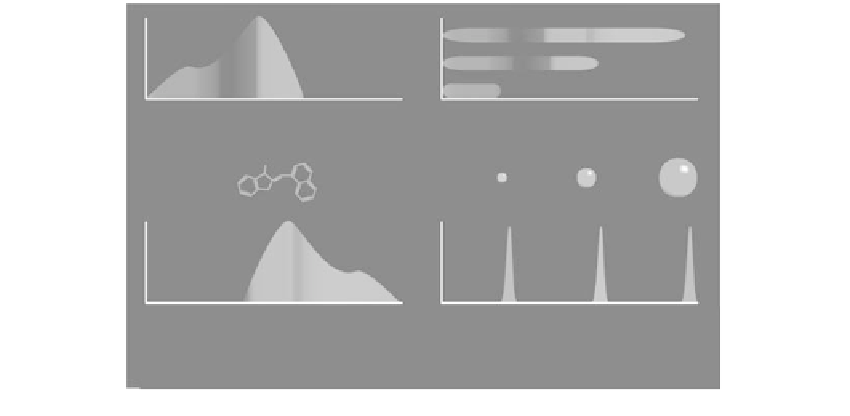
FIGURE 2.3
Aspects for consideration of selection of donor-acceptor pairs when developing energy transfer quenching sys-
tems. Panel A shows a representation of the broad band molecular excitation and emission that might be
observed for a fluorophore. The width of the spectral excitation band offers the opportunity for selection of a
wavelength that could provide selectivity for targeting a specific fluorophore in a mixture. The broad molecular
emission band is often undesirable, in that there can be spectral overlap with the absorption band of the same
molecular species, and any quenching partner would ideally need to have an absorption profile that would mir-
ror the emission band. Panel B is representative of the spectroscopy of quantum dots. Excitation can be done
using wavelength bands, or even full spectral excitation across the entire visible region. Emission is confined to
relatively narrow bands, and these can be tuned to appear at different wavelengths by selection of the diameter
of the quantum dot (size scale range of a few nanometers and higher). The narrow band of emission provides
advantages in selection of operating wavelengths to maximize energy transfer and to avoid background spectral
interference (when using narrow band excitation).