Information Technology Reference
In-Depth Information
a
b
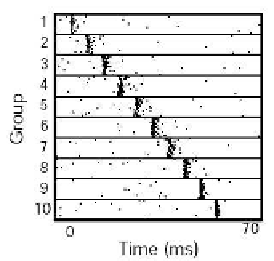
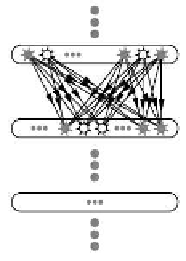
Fig. 13.1. Synre chain anatomy (a) and dynamics (b). (a) Chain of groups of neurons connected
in a feed-forward manner.
(b) Activity synchronizes further as it propagates along the chain.
(Modied from [38].)
feed-forward structures permit the stable propagation of synchrony [7, 38, 53, 62,
145, 159]. Model studies of synre chains that are actually embedded in recur-
rent networks (rather than treating the inuence of the embedding network just as
additional noise) show that persistent propagation of localized synchronous events
within recurrent cortical circuits is not simple to realize [83, 101, 146]. In particu-
lar, extensive numerical studies show that although propagation of synchrony can
be achieved, often pathological dynamics occur, for instance synchronous activity
which spreads and covers the entire network after a short time (`synre explosion'),
or synchronous activity in the embedded chain dies out quickly due to inhibition
from the embedding network [12, 101, 146]. It has been shown recently that depend-
ing on the features of individual neurons and on the network architecture, stable
propagation of synchrony along chains embedded in recurrent networks may also
be achieved in a robust way [83]. Another possibility to construct networks with
feed-forward pathways is to strengthen those connections that are already present
in a recurrent random network [164]. However, strong amplications of synapses
and specic changes in the response properties of neurons along the pathway are re-
quired to enable the propagation of synchrony over a few groups. A mechanism that
might enable persistent synchronous activity in embedded architectures with mod-
erately strong pathway structure is nonlinear enhancement of synchronous inputs
due to dendritic spikes that was recently found in neurophysiological experiments
([9, 46, 47, 116, 122], cf. also [106]).
Successive excitation of neurons in groups with distributed transmission delays
can generate spiking activity that is not synchronous, but precisely time-lagged
(with the lag dened by the transmission delays), resembling synre chain dynam-
ics. Works by Izhikevich and coworkers [68, 69] show that such groups of neurons
with strong coupling can spontaneously form in a random network due to spike tim-
ing dependent plasticity (see, e.g., [33, 79]) and that they generate detectable spike
patterns with millisecond precision although embedded in a larger network. Taken