Environmental Engineering Reference
In-Depth Information
Caniste
Drug substance
or
liquified propellants
excipients
Metering
Actuator
Atomization
orifice
Spacer
Actuator
mouthpiece
Space
mouthpiece
FIGURE 1.2
Basic components of a pMDI system.
More correctly, these devices should be called pressurized metered dose inhalers (pMDIs) to avoid
confusion with MDIs that incorporate and use dry powders and aqueous-based systems (discussed
in the following).
The irst MDI was commercialized in the mid-1950s to compete with glass nebulizers for the
delivery of asthma medications. The acceptance and utility of these delivery systems was quickly
established and they have now become the most common system for drug delivery to the respira-
tory tract. The basic design of pMDIs (Figure 1.2) has not changed greatly since their inception
and they typically contain three basic components: the active substance, the propellant system,
and other stabilizing excipients. These components are enclosed within an aerosol container with
a metering valve that connects to an actuator or aerosolization nozzle. An adaptor mouthpiece,
which may include one of a variety of holding chambers, allows the patient to draw the aerosol
into the lungs. The composition and design of pMDIs affects the performance of the drug delivery
system.
53
Propellants serve as a source of energy for atomization of the liquid formulation as it exits
the nozzle. They also function as a liquid phase for the dispersion of drug and other excipients that
often are present in suspension. Surfactants aid dispersion or dissolution in addition to providing
lubrication for valve components.
53-55
Solution formulations are typically attained by incorporation
of cosolvents such as ethanol.
53,56
Currently marketed products and those in development can be
divided into three classes based upon the propellant system used.
1.3.1.1 CFC Systems
Chloroluorocarbon (CFC) propellants have been the most common propellant type used in pMDIs.
These propellants include CFC 12 (dichlorodiluoromethane), CFC 11 (trichloroluoromethane), and
CFC 114 (1,2-dichloro-1,1,2,2-tetraluoroethane). The widespread use of these propellants was a
consequence of their low pulmonary toxicity, high chemical stability, purity, and compatibility.
53
Also, mixtures of these propellants can be formulated to yield desirable vapor pressures, densities,
and solvency properties for successful formulation of a variety of drug substances. However, pMDIs
containing CFCs have been phased out and are being replaced by other propellant systems. This is a
result of the linking of CFCs to the depletion of stratospheric ozone and the signing of the Montreal
Protocol on substances that deplete the ozone layer.
57,58
On April 14, 2010, the U.S. Food and Drug
Administration (FDA) issued a “inal rule” that conirms the fate of the last seven remaining pMDI
















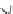















Search WWH ::

Custom Search