Information Technology Reference
In-Depth Information
relationship with a variety of invertebrate and vertebrate sea organisms, es-
pecially the Hawaiian sepiolid squid,
Euprymna scolopes
, and the Japanese
pinecone fish,
Monocentris japonica
[11]. In these symbiotic relationships, the
bacteria grows to densities of approximately 10
10
cells/l.
In the free-living state,
Vibrio fischeri
emits essentially no light (
<
0
.
8
photons/sec/cell). In the light organ of the Hawaiian sepiolid squid, however,
the same bacteria emit more than 800 photons/sec/cell, producing very visible
bioluminescence. In culture,
Vibrio fischeri
demonstrates a similar density-
dependent bioluminescence, with induction occurring at about 10
10
cells/l.
Work over many years has established that this behavioral change is due to a
natural mechanism of detecting cell densities, which has been termed
quorum
sensing
[5]. The quorum-sensing mechanism relies on the synthesis and de-
tection of a very specific, species-unique chemical, an
autoinducer
, which
mediates intercellular communications. In
Vibrio fischeri
, this autoinducer
chemical (VAI) has been identified as
N
-(3-oxohexanoyl)-3-amino-dihydro-
2-(3H)-furanone [2]. The gene,
LuxI
, catalytic protein, and synthetic pathway
for this chemical have also been identified [4].
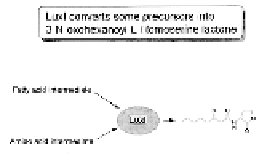
The
LuxI
gene encodes an acyl-homoserine lactone synthesase that uses
highly available metabolic precursors found within most Gram-negative pro-
karyotic bacteria—acyl-ACP from the fatty acid metabolic cycle, and
S
-adeno-
sylmethionine (SAM) from the methionine pathway—to synthesize VAI. The
VAI freely diffuses across the bacterial cell membrane. Thus, at low cell den-
sities, low VAI concentrations are available. Within a light organ, or at high
culture densities, VAI builds up within the environment, resulting in a density-
dependent induction of bioluminescence.
The response mechanism to VAI concentration has also been extensively
analyzed [13]. The
LuxR
gene codes for a two-domain DNA-binding protein
that interacts with VAI and the Lux box of the LuxICDABEG operon promoter
to exercise transcriptional control (Figure 7.13). At nanomolar concentrations,
VAI binds to the N-terminal domain of the LuxR protein, which in turn activates
(Light)
hv
(Light)
hv
LuxI
Luciferase
Luciferase
LuxR
P
luxR
luxI
luxC luxD luxA luxB luxE luxG
P
Regulatory Genes
Structural Genes
Figure 7.13
The lux operon (on the left), and luxI metabolism that catalyzes the for-
mation of
V. fischeri autoinducer
(on the right).