Biomedical Engineering Reference
In-Depth Information
d
n
4
t
3
n
g
|
1
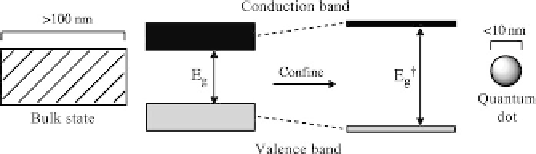
Figure 1.29
Increase in electronic band gap for quantum dot compared to bulk
material.
semiconductor. They function through a principle known as quantum
confinement where energy levels are dependent on the size of the particle. This
physics implies a band gap between the valance electron shell and the
conduction band, which is actually tunable according to the materials used for
fabrication and the dimension of the dot. For a semiconductor particle of the
size involved here the conventional band gaps associated with bulk materials
reduce to two discrete states which are termed quantum-confined levels. The
size effect leads to band gaps which are increased and, therefore, an increase of
input energy is required to excite electrons to the higher level (Figure 1.29). This
property in turn confers advantages on QDs with respect to fluorescence
spectroscopy, which results in an important application in biosensor-like
detection capability.
In contrast to the more conventional molecular fluorophores familiar to the
analytical chemist, QDs exhibit superior quantum yield, longer fluorescence
lifetime and the possibility for multiplexing (that is, excitation of different QDs
with the same wavelength of incident light). These properties are extremely
useful with respect to the detection of chemical and biochemical processes that
are instigated on the high surface area of quantum dots.
137
One example is the
use of the technology in a fluorescence resonant energy transfer (FRET) type of
detection experiment. FRET involves the use of donor-acceptor pairs in
proximity to produce fluorescence excitation and emission. In this case the QD
can be employed to function in tandem with a molecular fluorophore in order
to assay biochemical pairs.
137
An excellent case in point here is the detection of
nucleic acid duplex formation via excitation of a dye with the QD-based
emission; The core of the experiment is shown in Figure 1.30. Although the
strategy represents an extremely sensitive detection configuration the method
still requires a label, much as discussed above with respect to electrochemical
detection, is subject to the usual non-specific adsorption problems if employed
in complex media, and is dicult to use for time-dependent monitoring.
d
n
3
.
1.4.3.5 Raman Spectroscopy and the Metal Nanoparticle
In Raman spectroscopy a sample is conventionally irradiated with a laser light
source, usually at a wavelength removed for the particular absorbance band,
with the intensity of scattered radiation being measured with a spectrometer.