Biomedical Engineering Reference
In-Depth Information
d
n
4
t
3
n
g
|
1
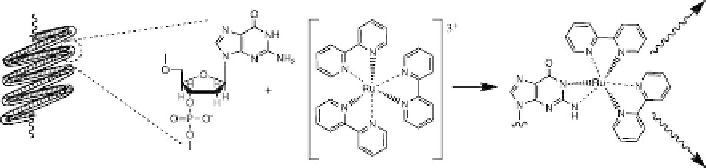
Figure 1.12
Ru(bpy)
3
21
label of nucleic acid strand as a precursor for electrochemical
detection of duplex formation.
so familiar to the diabetic patient. A further crucial aspect of all this work has
been, and continues to be, the possibility of using an electrochemical device for
in vivo assay of glucose in blood in an artificial pancreas scenario. However,
despite enormous effort over the years with the corresponding financial
implications, such a system is not available. The reason for this undoubtedly
lies in issues concerning sensor fouling in blood and on-the-fly calibration.
The detection of nucleic acid interactions and duplex formation at an
electrode surface by an amperometric protocol has been the subject of intensive
research for some time.
49
One example among several is use of the redox
properties of, for example, Ru complexes to enhance the electroactivity of
nucleic acid species (the intrinsic redox behavior of nucleic acid moieties is
insuciently sensitive for analytical purposes). The coupling of Ru(bpy)
3
21
mediated oxidation of the base guanine is said to lead to nucleic acid target
detection at least the attamole concentration (Figure 1.12). Curiously, the
electrochemistry community often describes this protocol as a 'label-free'
detection strategy which is obviously invalid given the necessary use of the Ru
adjunct agent. In more recent times scientists at the University of Toronto have
engineered this electrochemical approach into a hand-held device, ostensibly to
be employed for point-of-care assay of cancer.
50
Finally, with respect to amperometry, we mention the technique of
chronoamperometry, which involves the application of a square-wave potential
to the indicating electrode with measurement of the resulting steady-state
current over time. In this scenario, the concentration of an electroactive species
close to the electrode surface is reduced to zero. Accordingly, for elec-
trochemistry to take place the entity of interest must be transported to the
device by diffusion. The technique was employed some time ago to examine
neurotransmission and the electrochemical behavior of slices of brain tissue.
51
d
n
3
.
1.4.1.3 Impedance spectroscopy
Electrochemical impedance spectroscopy (EIS) has been available for more
than 30 years. In this technique a sinusoidally varying voltage is applied to an
electrochemical system and the resulting current is measured, usually over a
range of angular frequencies. Recording of these parameters can be employed
to compute the real and imaginary components of the electrical impedance (Z).