Biomedical Engineering Reference
In-Depth Information
category the ion selective (indicating) electrode (ISE) forms the basis of systems
capable of detecting species of biochemical interest.
41,42
By far the most
common one in use is the glass electrode, which is sensitive to changes in
hydronium ion concentration. This type of electrode is often termed a
membrane electrode in view of the development of a junction potential
connected to selective ion exchange processes at the membrane-solution
interface. Other systems available for ion selective detection include electrodes
which employ crystalline matrices, e.g. LaF
3
for F
, and those which incor-
porate liquid ion exchangers in polymer matrices such as systems for sensing
Ca
21
. A typical general equation that gives the potential, E, of an electrode for
an ion, a, in the presence of an interfering species, b, is:
d
n
4
t
3
n
g
|
1
E
¼
E
0
ð
RT
=
xF
Þ
log
ð
a
a
þ
Ka
;
b
a
b
Þ
ð
1
:
2
Þ
where R is the gas constant, T is temperature, F is the Faraday constant, x is
the number of electrons involved in the electrochemical process, a
a
and a
b
are
the activities of the two ions, and K
a,b
is a selectivity coecient for the
electrode.
The ISE has been the basic structure used to develop enzyme-based
molecular sensors for a number of years.
42
In this type of device the analyte is
allowed to interact with an immobilized enzyme resulting in conversion to a
product which can be detected by the electrode. The product of the catalyzed
reaction can be a gas such as ammonia or a cation such as the hydronium ion.
In either case the electrode actually detects a change in pH in terms of response.
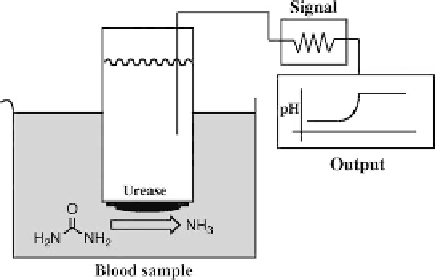
A simple example of this approach is the electrode for sensing blood urea via
the enzyme, urease (Figure 1.9). The product of the reaction, NH
3
, alters the
localized pH which is then detected by a glass electrode.
An alternative transducer to the conventional electrode outlined above is
the field-effect transistor (FET), which was introduced by Bergveld
43
in the
1980s for ion sensing. Since this device can be integrated into various circuits
in a miniaturized format and has been employed to a significant degree with
d
n
3
.
Figure 1.9
A urease-based enzyme electrode. Note device detects changes in pH as a
consequence of enzyme activity.