Biomedical Engineering Reference
In-Depth Information
d
n
4
t
3
n
g
|
7
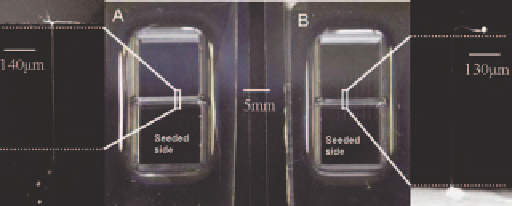
Figure 3.12
(A) Image of the planar glass device with channeled PDMS mold.
(B) Planar PDMS with channeled glass—the zoom images show the cells
growing through the channel into the adjacent compartment.
48
(Reprinted by kind of permission of the Royal Society of Chemistry.)
chemoanity of tip of the neurite plays an important role in directing the
growth of neurites along specific pathways.
51
Neuronal wiring and guidance is
a response to multiple attractive and repulsive cues operating at various spatial
scales. Furthermore, there are elaborate time sensitive regulatory mechanisms
which dictate whether a given cue elicits a response from the neurite or is
ignored.
52
The concentration and gradient of growth factor ingredients are
essential in providing guidance cues for neurite response.
53
Microfluidic devices
are an important tool for studying neurite guidance under chemogradients as
well as the tuning and promotion of neurite regeneration.
54
The power of microfluidic devices to control the microenvironment around
the neurons offers an experimental in vitro platform for studies that seek to
investigate the multitude of growth cone cues. Microfluidic devices offer
distinct advantages over other in vitro systems for studies investigating the
effects of growth factors on neurite outgrowth.
55-57
Through use of micro-
channels and compartments, microfluidic devices offer user-defined control
over the spatio-temporal distribution of growth factor in the extracellular
matrix environment. Furthermore, by isolating and tracing individual neurites
as they grow, these devices allow identification of cell-specific processes beyond
the global response of the neuronal population to specific cues. These
advantages can elucidate the complexities of physiologically relevant growth
factor gradients and their specific cell signaling processes.
Aside from localized studies and compartmentalization of cells, use of
microfluidic structures and valves have made cell co-culture platforms possible,
where individual manipulation of the microenvironment of different cell types
can be performed on the same experimental setup. The application of these
microstructures enables new experimental processes to precisely control and
manipulate the microenvironment of cells.
58
These co-culture platforms have
been utilized to maintain healthy cultures of hippocampal neurons and glia for
several weeks under optimal conditions.
59
The microfluidic structures used in co-culture experiments rely on chambers
with interconnected channels with dynamically controlled connections. An
example of such structures is shown in Figure 3.13 where a structure is based on
n
3
.