Geology Reference
In-Depth Information
Ignition
Heat Loss
Heat Gain
Temperature,
°
C
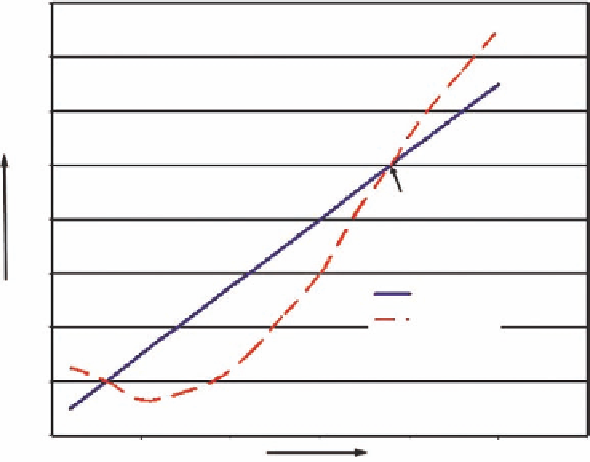
Figure 16.3.1. An example illustrating the rate of heat gain versus the rate of heat loss as a function of increasing
temperature. Spontaneous combustion occurs at the ignition point. From Kim and Chaiken 1993, p. 7.
probably more prevalent in the Western outcrop fires. Spontaneous combustion in adjacent materials is also a
significant cause of AML fires, since abandoned strip mines and areas where the coal outcrop has been stripped are
frequently used as local trash dumps. Spontaneous combustion in the trash will ignite the coal refuse or the outcrop.
Such fires may have no visible indications until the fire is well established.
Spontaneous Combustion
Spontaneous combustion in the coal or coal refuse is related to the oxidation of the coal to form CO
2
, CO, and H
2
O.
The oxidation of pyrite and the adsorption of water on the coal surface also are exothermic or heat-generating
reactions that increase the probability of spontaneous combustion (Kim, 1977). Thermophilic bacteria may also
contribute to raising the temperature of the coal. In waste banks, most of the oxygen diffusing from the surface is
consumed by bacterial activity within a meter. However, enough oxygen is available at depth to support
combustion.
To estimate the spontaneous combustion when coal is stored underground in mines, 46 samples of various ranks
were tested in an adiabatic calorimeter (Elder et al., 1945). The rank of the coal, the temperature at which it was
stored, the particle size, moisture and ash content, and the availability of oxygen were the primary factors
influencing the probability of spontaneous combustion during storage.
The self-heating temperatures of 29 coals were determined in an adiabatic-type calorimeter (Kuchta et al.,
1980). The data in this experiment indicated that lignite and subbituminous coals can self heat at tempera-
tures as low as 30°C, but bituminous coals require a temperature of 60°C or more. The self-heating tendency
of a coal mass can vary with its porosity, moisture content, ventilation rate, and with the humidity of the
ventilating air.
In sealed flask experiments, the CO index,COemission,andO
2
absorption were found to decrease with
increased coal rank. Although these are sensitive to increased temperature, the results of sealed flask experi-
ments were not considered directly transferable to the open environment of a coal mine. In another test, the
relative self-heating tendencies of six coals, from high-volatile C to low-volatile bituminous in rank, the
minimum self-heating temperatures varied from 35°C for the lower rank coals to 105°C for the high-rank coal
(Miron et al., 1990). The minimum self-heating temperature was correlated to the amount of O
2
adsorbed by the
undried coal samples.