Geology Reference
In-Depth Information
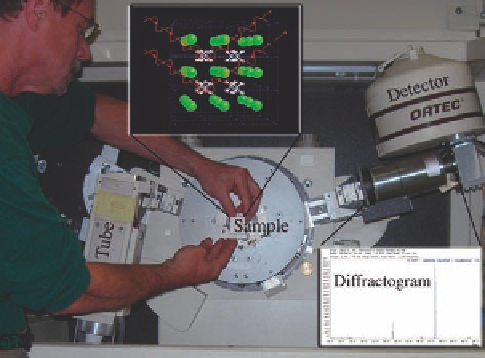
Figure 10.1.2. X-ray diffractometer arrangement. The X-ray source (tube) generates monochromatic radiation that
is focused on the sample. Upper inset shows the ideal crystal structure of salammoniac (NH
4
Cl, N= light blue,
H= pink, and Cl = green) with waves schematically entering and scattering off of atomic planes according to
Bragg
s law. Blue lines outline the edge (3.89 Å) of the repeating unit cell. Lower right inset shows the diffraction
pattern (diffractogram) obtained by scanning over a wide range of angles and detecting the coherent-constructive
interference of the X-rays. The pattern is unique to the types of atoms in the unit cell, shape of the unit cell, and
geometry of the X-ray beam path. Photo by Paul A. Schroeder, 2007
'
.
The combination of (1) the diffractometer geometry (goniometer positions) and X-ray wavelength, (2) the location
and types of atoms in the unit cell, and (3) the shape of the unit cell results in each mineral producing a unique
pattern of diffracted X-rays. These relationships were established by the physicist W.L. Bragg in 1912 and are
summarized in Bragg
'
s law,
λ
= 2dsin
θ
, where
λ
is the radiation wavelength, d is the spacing between repeating
crystal planes, and
θ
is the angle of X-ray incidence. The detected signal is sometimes referred to as the
“
reflected
beam.
It is like reflected light in the sense that the angle of incidence equals the angle of scattering. It is unlike
light in that light will reflect at all angles, but diffracted X-rays will only
”
“
reflect
”
under the Bragg condition.
Diffraction patterns are therefore plotted using 2
on the horizontal scale. Searching computer libraries of known
diffraction data (i.e., patterns), such as the Powder Diffraction File (PDF) available from the International Centre
for Diffraction Data (http://www.icdd.com) or the American Mineralogist Crystal Structure Data Base (http://www.
minsocam.org), allow one to closely match an unknown pattern with that of a known mineral (Figure 10.1.3).
θ
The diffraction pattern search and match process, however, is not always simple and complete matches are difficult
because of natural variability in a mineral
s crystal structure and chemistry. So, do not expect a computer search
and identification process to be fool proof if its search algorithms are not designed for complex mixtures. There are
many other factors that can lead to a discrepancy between database diffraction patterns and the pattern of an
unknown sample of interest (Hurst et al., 1997). It is also possible that there are new minerals to be discovered in
association with coal-fire gas vents; not in the current databases. Finally, the sensitivity or detection limit of XRD is
dependent upon the crystallinity of the material. If a mineral has a highly disordered crystal structure and is in low
abundance, then the XRD method may not uniquely detect the mineral, even in concentrations of up to 5
'
10% by
weight. This is in sharp contrast to the EMPA method (discussed below), which can clearly image and chemically
analyze a small mineral grain distinctive in its crystal shape and appearance, yet in very low abundance. Moore and
Reynolds (1989) and Hurst et al. (1997) are good sources for learning more about X-Ray diffraction.
-
Table 10.1.1contains a list of coal-fire sites around the world where sulfur-bearing mineral assemblages nucleated
from the gas. Although the collective mineral assemblages from each site are not exactly the same, there is a
commonality of hydrated sulfate minerals. In many cases, the ammonium cation (NH
4
+
) is a common constituent,
likely derived from the volatilization of nitrogen in the coal under anoxic conditions.
When mineral assemblages from coal fires and volcanic hot springs are compared (Table 10.1.1), it is readily seen
that similar NH
4
+
and Fe-bearing hydrated sulfate minerals are present. Their differences are stoichiometric or
seen in the proportions of K, Ca, Fe, Mg, and Al in the hydrated sulfates. These distinctions can be attributed to the
difference between the silicate mineralogy of volcanic host rocks (as volcanics are quite variable in composition)
and the mineralogy of the sedimentary rocks hosting the coals. For example, clastic sedimentary rocks tend to be