Geology Reference
In-Depth Information
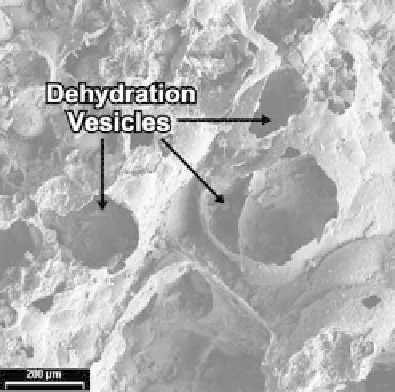
Figure 9.1.4. GLAS process revealed by a cryptocrystalline mass of millosevichite and alunogen from a gas vent
in the Wuda coalfield, Inner Mongolia (SEM). Vesicular texture and Al ions in the mineral assemblage, collected
from a quartzofeldspathic sandstone substrate, suggest reaction (9.1.5) occurred, as discussed in the text. From
Stracher et al., 2005a, with permission of the Mineralogical Society of America.
where the mineral Z(s) is the dehydration or hydration product of Y(s). In the Wuda coalfield of Inner Mongolia, a
sample encrusting a gas vent was collected from quartzofeldspathic sandstone. The sample is vesicular (Figure
9.1.4) and contains both alunogen, Al
2
ð
SO
4
Þ
3
. The vesicles, Al ions
likely acquired from the sandstone substrate, and the simultaneous occurrence of these minerals suggest that
process (9.1.5) occurred at the vent (Stracher et al., 2005a). The occurrence of both millosevichite and alunogen
and the vesicles suggest the dehydration reaction
SO
4
Þ
3
⋅
17H
2
O, and millosevichite,
ð
Al
Þ
2
ð
Al
2
ð
SO
4
Þ
3
⋅
17H
2
O
→
Al
2
Millosevichite
þ
ð
SO
4
Þ
3
17H
2
O
↑
Alunogen
The vesicular texture may have developed as liquid water or water vapor escaped from the assemblage. In
addition, the incomplete breakdown of alunogen, evident from its occurrence with millosevichite, is the result
of a temperature decrease subsequent to the increase that promoted dehydration. As suggested by Stracher
et al. (2005a), the fluctuating temperatures and concomitant effects on the assemblage are accounted for by
variations in combustion and atmospheric conditions and possibly removal (temperature decrease) of the
assemblage from the vent during sample collecting. It is possible that the reaction path involving the
vesicular assemblage found at Wuda is more complicated than that presented above. For example, mill-
osevichite may have initially formed at the vent and in the presence of water vapor exhaled at the surface or
atmospheric moisture, hydrated to alunogen as the vent
temperature fell, followed by the incomplete
dehydration of this mineral as described above.
Gas-Liquid Precipitation (GLP) occurs when a gas first condenses to a liquid from which a solid precipitates,
followed by solidification of the remaining liquid. Described by Wagner and Ellis (1964), the first and only
recorded
occurrence of this process (Figure 9.1.5) was observed by Finkelman et al. (1974) in association
with burning anthracite in Forestville, Pennsylvania. According to Finkelman et al. (1974) and Lapham et al.
(1980), sulfur droplets condensed from a S
2
(g) component in the anthracite gas. The droplets then acted as a
catalyst and absorbed Ge from the gas to form a S-Ge solution. Once the solution was saturated in Ge, germanium
sulfide (GeS
2
) precipitated. The process resulted in the nucleation of elongate GeS
2
crystals capped by bulb-like
amorphous sulfur, solidified from the remaining liquid sulfur, S(l), droplets at the end of the GeS
2
crystals. The
process is summarized by the reactions
natural