Geography Reference
In-Depth Information
The total potential energy is not a very useful measure of energy in the atmo-
sphere because only a very small fraction of the total potential energy is available
for conversion to kinetic energy in storms. To demonstrate qualitatively why most
of the total potential energy is unavailable, we consider a simple model atmosphere
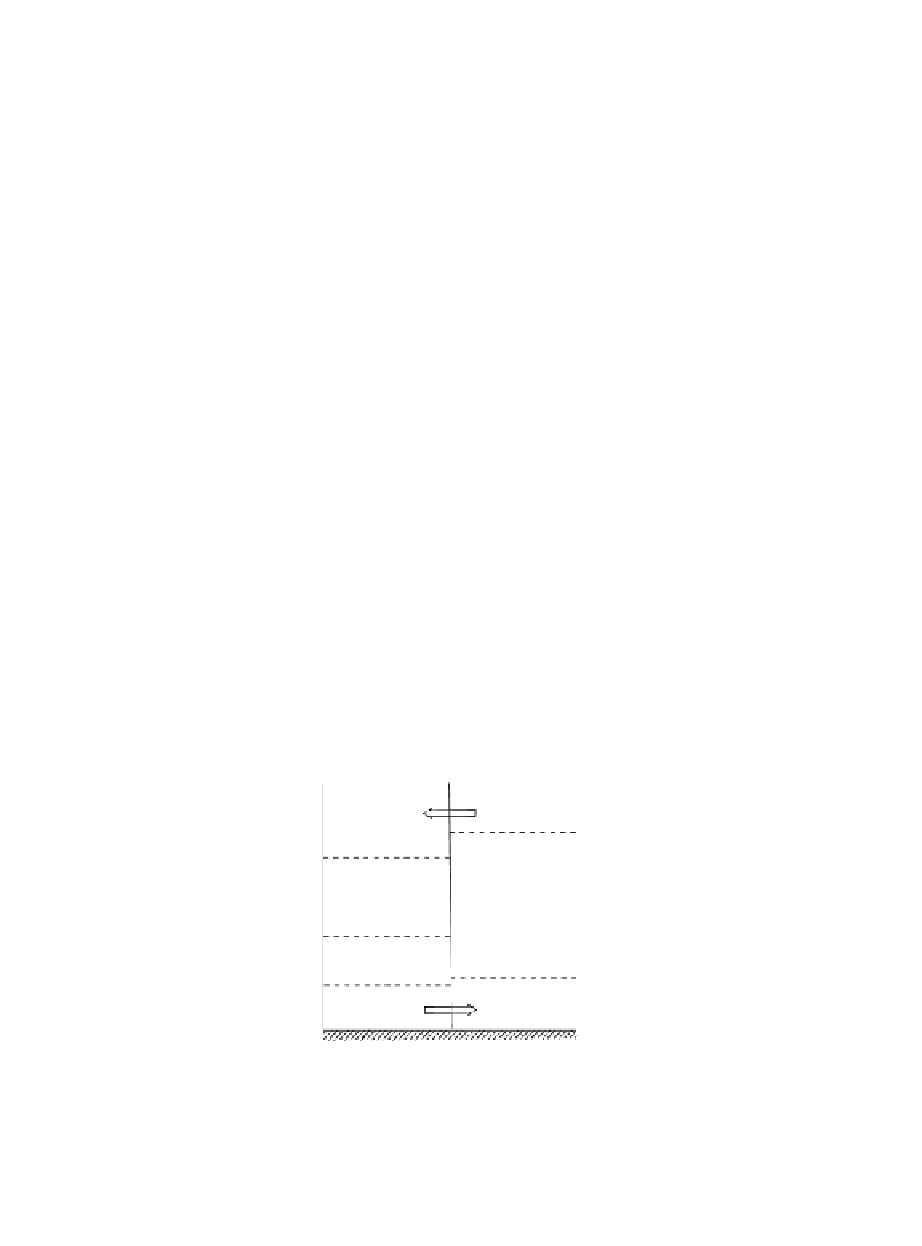
that initially consists of two equal masses of dry air separated by a vertical partition
as shown in Fig. 8.7. The two air masses are at uniform potential temperatures θ
1
and θ
2
, respectively, with θ
1
<θ
2
. The ground level pressure on each side of the
partition is taken to be 1000 hPa. We wish to compute the maximum kinetic energy
that can be realized by an adiabatic rearrangement of mass within the same volume
when the partition is removed.
Now for an adiabatic process, total energy is conserved:
E
K
+
E
P
+
E
I
=
constant
where E
K
denotes the kinetic energy. If the air masses are initially at rest E
K
=0.
Thus, if we let primed quantities denote the final state
E
K
+
E
P
+
E
I
=
E
P
+
E
I
so that with the aid of (8.32) the kinetic energy realized by removal of the partition
may be expressed as
E
I
Because θ is conserved for an adiabatic process, the two air masses cannot mix.
It is clear that E
I
will be a minimum (designated by E
I
) when the masses are
rearranged so that the air at potential temperature θ
1
lies entirely beneath the air
at potential temperature θ
2
with the 500-hPa surface as the horizontal boundary
between the two masses. In that case the total potential energy (c
p
/c
v
)E
I
E
K
=
c
p
/c
v
E
I
−
is not
(hPa)
250
θ
2
θ
1
z
500
750
1000
Fig. 8.7
Two air masses of differing potential temperature separated by a vertical partition. Dashed
lines indicate isobaric surfaces. Arrows show direction of motion when the partition is
removed.