Biology Reference
In-Depth Information
One of the most vexing problems in examining cell-cell interactions is to
precisely measure the adhesion force between contacting cells—that is, the
amount of force necessary to separate adjacent cells—in the cells' natural
environment. Three common techniques to approximate this measurement
are double micropipette aspiration (
Shao et al., 2004
) (or by adapting micro-
plates or similar methods), cell aggregate assays, and cell sheet assays. Double
micropipette aspiration relies on partially aspirating a cell in each of two
pipettes, allowing the cells to contact, and then aspirating one of the cells
to separate them. Cell aggregate assays rely on the use of a hanging drop cul-
ture (or similar mechanism) to generate a small cluster of cells which do not
adhere to a substrate, which are then collected and sheared, often by
pipetting or vortexing, to measure aggregate cohesion strength (
Benjamin
et al., 2010; Franzen et al., 2012
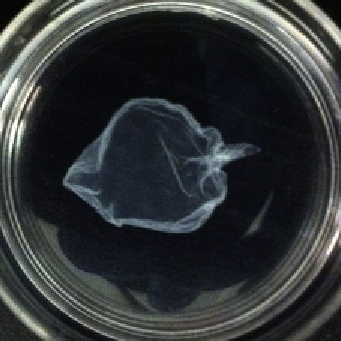
). Cell sheet assays use confluent monolayers
of cells (
Fig. 5.3
). In most cases, the monolayer is dissociated from the surface
using enzymatic compounds like dispase and then sheared (
Franzen et al.,
2012; Wei et al., 2011
). Alternative methods of dissociating the monolayer,
such as thermoresponsive polymers or soluble matrix could also be used
(
Akiyama et al., 2004; Tsuda et al., 2004
). A deform-drag method may
be used to assess the relative strength of cell-cell to cell-matrix adhesion,
although it appears to be best applied to cell types having modest cell-matrix
adhesion strength (
Huang et al., 2008
).
Figure 5.3 A cell sheet in a 35-mmdish, lifted by dispase, is generally fragile and difficult
to manipulate. Since it is effectively in suspension, it will move around and fold on itself
if not handled carefully. Such difficulties (and others) impede full characterizations of
cell sheet properties and cell
-
cell interactions.

Search WWH ::

Custom Search