Biology Reference
In-Depth Information
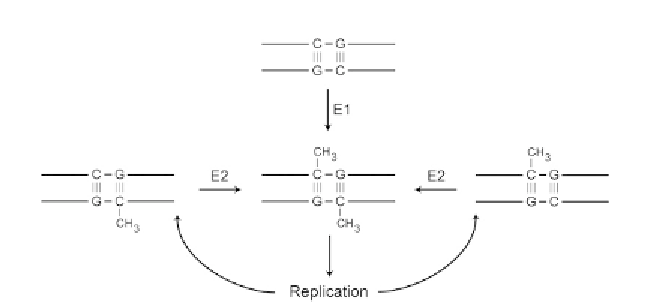
Figure 1.2 Postulate of dual enzymatic activity leading to perpetuation of stably
modified DNA. E1 designates a de novo methyltransferase enzymatic activity acting
on unmethylated DNA. E2 designates a maintenance methyltransferase activity acting
on hemimethylated DNA soon after DNA replication. After
Holliday and Pugh (1975)
.
are generated by DNA replication and then enzymatically acted on to con-
vert them to fully methylated DNA. In contrast, because cytosines are not
found on the opposite strands from asymmetric CpHpH sites, such sites can-
not be substrates for maintenance methyltransferase activities. How methyl-
ation is placed on either symmetric or asymmetric sequence targets is
addressed below.
2.4. Experimental support for maintenance
methyltransferase activity
The two main lines of evidence for
bona fide
cellular maintenance methyl-
transferase activity (conversion of hemimethylated DNA to fully methylated
DNA) come from biochemical and developmental studies of the mamma-
lian DNMT1 enzyme. DNMT1 protein can catalyze the transfer of a methyl
group to cytosine on both unmethylated double-stranded DNA templates
and hemimethylated DNA templates, consistent with two possible
models. Either DNMT1 indiscriminately targets unmethylated CpG dinu-
cleotides, regardless of their location on unmethylated or hemimethylated
DNA. Alternatively, DNMT1 preferentially recognizes and methylates
hemimethylated DNA, and has very little if any activity on fully
unmethylated DNA. Indeed, comparisons of the full-length and a truncated
version of DNMT1 containing just the C-terminal catalytic domain indi-
cated that the truncated form fails to discriminate effectively between
unmethylated and hemimethylated templates, whereas full-length DNMT1
methylates hemimethylated DNA much more effectively (11-fold) than
Search WWH ::

Custom Search