Geology Reference
In-Depth Information
Chlorine Chemistry in Stratosphere
CH
3
Cl
h
ν
CFCl
3
ClONO
2
OH
O
3
h
ν
NO
2
NULL CYCLE
Cl
ClO
NO
HO
2
O(
3
P)
Loss cycles
OH
HOCl
h
ν
HCl
ACTIVE SPECIES
RESERVOIRS
SOURCE GASES O
3
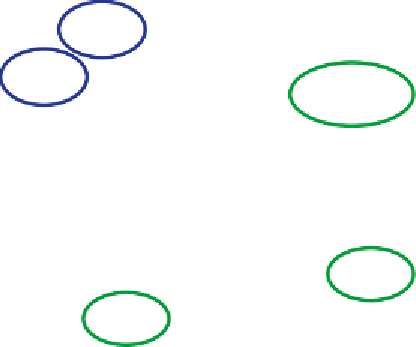
Figure 25
Inorganic chemistry involved in the interconversion chlorine species in the
stratosphere. The relationship between source gases, active species and reser-
voir species is illustrated
HO
x
,NO
x
and ClO
x
are present in the natural atmosphere but in the
contemporary atmosphere these have been supplemented by anthro-
pogenic sources. For example, with the chlorine species the most
abundant natural precursor is methyl chloride, CH
3
Cl. There is little
interhemispheric difference in the concentration of CH
3
Cl indicative of
a strong oceanic source, though there is a rising contribution for
biomass burning as well as small contribution from volcanic emission.
52
As CH
3
Cl has a tropospheric lifetime of a year, it can be transported to
the stratosphere where on reaction with OH or via photolysis it can
release the chlorine. Figure 26 shows the primary sources of chlorine
species for the stratosphere separating the anthropogenic and biogenic
source categories.
There are a number of potential sources of anthropogenic pollution
that could find there way to the stratosphere. One of the first problems
that scientists were worried about was Concorde or stratospheric su-
personic transport (SST). Aircraft engines produce large amounts of
nitrogen oxides (e.g. NO) on combustion of the fuel. Injection of these
nitrogen oxides directly into the atmosphere could supplement the
relevant catalytic cycle plus they can be very long-lived at high altitudes.
Initial estimates suggested that SST would lead to substantial losses of