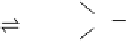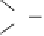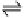Geology Reference
In-Depth Information
significant concentration of H
2
O
2
present in solution. The oxidation
proceeds as
O
−
ð
2
:
72
Þ
HSO
−
+ H
2
O
2
S
OOH
+
H
2
O
O
O
−
ð
2
:
73
Þ
H
2
SO
4
+ A
−
S
OOH
+ HA
O
where HA is an acid. The ubiquitous occurrence of H
2
O
2
, its solubility,
its high reactivity and pH independence (under atmospheric conditions)
of the rate constant for the reaction with SO
2
makes H
2
O
2
one of the
most important oxidants for SO
2
in the troposphere. A more detailed
description of aqueous-phase oxidation of SO
2
is given in ref. 36.
2.9 HALOGEN CHEMISTRY
In comparison to the atmospheric chemistry taking place in the strat-
osphere where halogen chemistry is well known and characterised (see
Section 2.10), there has been much debate as to the role of halogen
species in the oxidative chemistry of the troposphere. There is growing
experimental evidence as to the prevalence of halogen chemistry as part
of tropospheric photochemistry (see Table 6).
14,37
Much of the proposed
halogen chemistry is propagated through the reactions of a series of
halogen atoms and radicals.
Table 6 Sources of reactive halogen species found in various parts of the
troposphere
Species, site
Likely source mechanism
ClO
x
in the polar
boundary layer
By-product of the ''Bromine Explosion''
ClO
x
by salt flats
By-product of the ''Bromine Explosion''
BrO
x
in the polar
boundary layer
Autocatalytic release from sea-salt on ice, ''Bromine explosion''
mechanism
BrO
x
in the Dead sea
basin
''Bromine Explosion'' (salt pans)
BrO
x
in the free
troposphere
(1) Photo-degradation of hydrogen-containing organo-halogen
species (e.g. CH
3
Br), (2) ''Spill-out'' from the boundary
layer, (3) Transport from the stratosphere?
BrO
x
in the MBL
''Bromine Explosion'' mechanism
IO
x
in the MBL
(1) Photo-degradation of short-lived organo-halogen species
(e.g. CH
2
I
2
) (2) Photolysis of I
2
?
Source: After ref. 37.