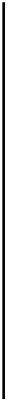Geology Reference
In-Depth Information
NO
2
þ
OH
!
HNO
3
k
2
¼
1
:
4
10
11
cm
3
molec s
1
ð
7
:
7
Þ
Making the crude assumption of a constant concentration of OH
radical
6
(more justifiable for the long-lived methane, for which fluctu-
ations in OH will average out, than for short-lived nitrogen dioxide)
d
dt
½
CH
4
¼
k
2
½
CH
4
½
OH
¼
k
0
1
½
CH
4
where k
0
1
¼
k
2
[OH]
Worked example
What are the atmospheric lifetimes of CH
4
and NO
2
if the diurnally
averaged concentration of OH radical is 1
10
6
molec cm
3
?
k
0
1
¼
6
:
2
10
15
1
10
6
¼
6
:
2
10
9
s
1
Then from Equation (7.4)
t
¼
k
1
¼ð
6
:
2
10
9
Þ
1
s
¼
5
:
1 years for CH
4
By analogy, for nitrogen dioxide, the lifetime
t
¼
20 h
This general approach to atmospheric chemical cycling has proved
useful in many instances. For example, measurements of atmospheric
concentration, [A], for a globally mixed component may be used to
estimate source strength, since
S
0
¼
R
0
¼
d
½
A
dt
¼
k
2
½
A
½
OH
and
S
¼
S
0
V
where V is the volume of atmosphere in which the component is mixed.
Source strengths estimated in this way, for example, for the compound
methyl chloroform, CH
3
CCl
3
, known to destroy stratospheric ozone,
may be compared with known industrial emissions to deduce whether
natural sources contribute to the atmospheric burden.