Geology Reference
In-Depth Information
LEAD
100
0
1800
1900
2000
Date of deposition of snow or ice
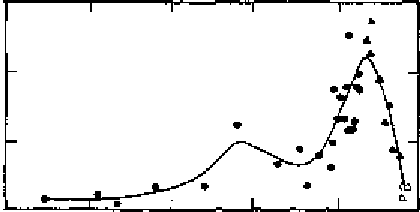
Figure 2 Changes in lead concentrations in snow/ice deposited in central Greenland from
1773 to 1992
(Adapted from Candelone et al.
4
)
when considering the behaviour of trace components. For example, a
highly reactive hydrocarbon emitted at ground level will probably be
decomposed in the boundary layer. Sulfur dioxide, with an atmospheric
lifetime of days, may enter the free troposphere but it is unlikely to enter
the stratosphere. Methane, with a lifetime of several years, extends
through all of the three regions.
It should be noted from Table 1 that the atmosphere is a much smaller
reservoir in terms of mass than the others. The implication is that a given
pollutant mass injected into the atmosphere will represent a much larger
proportion of total mass than in other reservoirs. Because of this, and
the rather rapid mixing of the atmosphere, global pollution problems
have become serious in relation to the atmosphere before doing so in
other environmental media. The converse also tends to be true, that once
emissions into the atmosphere cease, or diminish, the beneficial impact is
seen on a relatively short timescale. This has been seen in relation to
lead, for instance, where lead in Antarctic ice (derived from snow) has
shown a major decrease resulting from diminishing emissions from
industry and use of leaded petrol
4
(Figure 2). Improved air quality in
relation to CFCs will take longer to achieve because of the much longer
atmospheric lifetimes (4100 years) of some species (see Chapter 1).
7.1.2 Lifetimes
A very useful concept in the context of pollutant cycling is that of the
lifetime or residence time of a substance in a given reservoir. We can
think in terms of substances having sources, magnitude S, and sinks,
magnitude R. At equilibrium
R
¼
S