Geology Reference
In-Depth Information
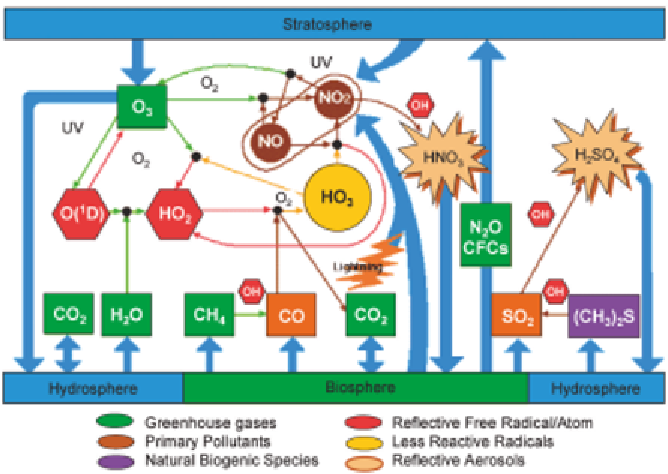
Figure 8 A simplified scheme of tropospheric chemistry. The figure illustrates the
interconnections in the chemistry, as well as the role of sources, chemical
transformation and sinks
The fate of the bulk of the O(
1
D) atoms produced via reaction (2.7) is
collisional quenching back to ground-state oxygen atoms, viz
O(
1
D)
þ
N
2
O(
3
P)
þ
N
2
-
(2.9)
O(
1
D)
þ
O
2
O(
3
P)
þ
O
2
-
(2.10)
The fraction of O(
1
D) atoms that form OH is dependent on pressure and
the concentration of H
2
O; typically in the marine boundary layer (MBL)
about 10% of the O(
1
D) generate OH. Reactions (2.7 and 2.8) are the
primary source of OH in the troposphere, but there are a number of
other reactions and photolysis routes capable of forming OH directly or
indirectly. As these compounds are often products of OH radical initi-
ated oxidation they are often termed secondary sources of OH and
include the photolysis of HONO, HCHO, H
2
O
2
and acetone and the
reaction of O(
1
D) with methane (see Figure 9). Table 2 illustrates the
average contribution of various formation routes with altitude in a
standard atmosphere.
Two important features of OH chemistry make it critical to the chem-
istry of the troposphere. The first is its inherent reactivity; the second is its