Geology Reference
In-Depth Information
produce a soil of pH between 7 and 8.5, depending upon the concentra-
tions of CO
2
and Ca
21
or Mg
21
ions. In arid regions, the lack of rainfall,
and hence leaching, allows the more soluble Na
2
CO
3
to accumulate and
the pH rises to about 10.5.
5.4.4 Influence of pH on Soils
A major influence of pH on soil is its effect on biological activity. Many of
the soil microorganisms function within a narrow optimal range of pH
and their activity is inhibited in more acidic or alkaline conditions. For
example, the nitrifying bacteria mentioned above, Nitrosomonas and
Nitrobacter, have a pH optimum in the range 6-8, and are severely
inhibited below pH 5.5. Conversely, the iron- and sulfur-oxidizing
bacteria of the genus Thiobacillus are active only under acid conditions,
and are inhibited at pH 4 5. In general, bacteria are less tolerant of acid
conditions than fungi. Soil animals too are affected by pH; earthworms,
for example, cannot survive below a pH of about 4.5.
Certain soil components have a variable, pH-dependent charge. The
charge on the humified soil organic matter is negative overall due to
dissociation of carboxyl and phenolic groups, but its magnitude varies,
being greater at high pH (see Section 5.3). The hydrous oxides and edges
of clay minerals are positively charged at low-pH values and negative at
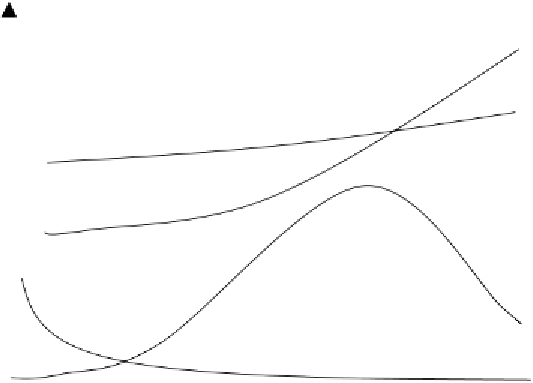
Ca, Mg
N, S
P
Mn, Fe, Cu, Zn
4.0
8.0
pH
Figure 11 Relative availabilities of plant nutrients in soil as affected by pH (only relative
amounts of individual nutrients should be compared)