Geology Reference
In-Depth Information
mineral are illitic in nature, while others more closely resemble vermi-
culite or smectite. Weathering at the edges of the clay mineral can also
lead to changes. For example, at the edge of an illite crystal, there may
be some loss of interlayer K
1
ions and their replacement by other
cations. This results in a widening of the interlayer space to form the so-
called wedge site (Figure 6).
5.2.3.2 Short-Range Order (Amorphous) Aluminosilicates. This is a
group of minerals that have small particle size and no regular crystal
structure, and as a result produce poorly defined X-ray diffraction
patterns. They do, however, show some regular short-range structure.
Thus, there has been a tendency over recent years to describe them as
short-range order, rather than amorphous, aluminosilicates. They have
a very high surface area, making them highly reactive. They have both a
K
+
K
+
K
+
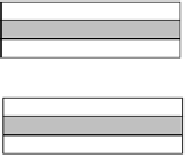
Kaolinite
Illite
Mg
2+
H
2
O
Mg
2+
Ca
2+
H
2
O
H
2
O
Ca
2+
Mg
2+
Ca
2+
H
2
O
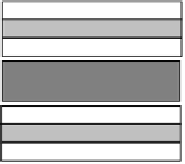
Vermiculite
Smectite
Chlorite
Figure 6 Structures of common clay minerals
Silica tetrahedral sheet;
alumina
octahedral sheet;
brucite sheet.