Geology Reference
In-Depth Information
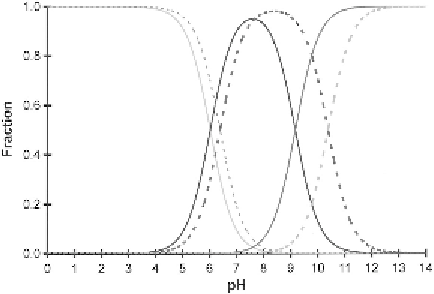
Figure 9 The distribution of carbonic acid species at 20 1C as a function of pH in seawater
of salinity 35(solid lines) and pure water (dotted lines)
it is necessary to consider in more detail the factors influencing the
inorganic carbon cycle. Two useful parameters can be introduced. Firstly,
the total concentration of inorganic carbon,
P
CO
2
, in seawater is
P
CO
2
¼
[CO
2
]
þ
[H
2
CO
3
]
þ
[HCO
3
]
þ
[CO
3
2
]
The first term is negligible and as evident in Figure 9, the major species
at pH 8 are HCO
3
and CO
3
2
.
Alkalinity is defined as a measure of the proton deficit in solution and
should not to be confused with basicity. Alkalinity is operationally
defined by titration with a strong acid to the carbonic acid end point.
This is known as the titration alkalinity (TA). Seawater contains weak
acids other than bicarbonate and carbonate that are titrated, and
therefore TA is given as
TA
¼
[HCO
3
]
þ
2[CO
3
2
]
þ
[B(OH)
4
]
þ
[OH
]
[H
1
]
This is a simplified version of the more general expression in Chapter 3. The
influence of [OH
]and[H
1
] on the TA is small and can often be ignored.
The borate contributes about 3% of the TA, and if not determined
independently, can be estimated from the apparent boric acid dissociation
constants and the salinity, relying upon the relative constancy of compo-
sition of sea salt. This correction would give the carbonate alkalinity (CA)
CA
¼
[HCO
3
]
þ
2[CO
3
2
]
Considering the dissociation constants above, this can be alternatively
expressed as
CA
¼
K
0
1
H
2
CO
3
H
fg
þ
2 K
0
1
K
0
2
H
2
CO
3
½
½
ð
4
:
11
Þ
H
f
2