Geology Reference
In-Depth Information
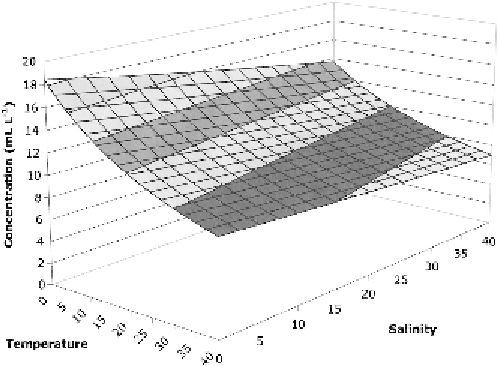
Figure 7 The solubility of nitrogen in seawater as a function of temperature and salinity
equilibrated at different temperatures were mixed, then the resulting
water body would be supersaturated. Thirdly, gases like oxygen that are
produced in situ by biological activity may become supersaturated,
particularly when evasion to the atmosphere is hindered.
The gas solubility for a water body in equilibrium with the overlying
air mass can be expressed in several ways. It is convenient to consider
Henry's Law that states
H
¼
c
a
c
w
1
where H is the Henry's law constant and c
a
and c
w
refer to the
concentration of a gas in air and water, respectively. As discussed by
Liss (1983),
12
air-sea exchange occurs when a concentration gradient
exists (i.e., DC
¼
c
a
H
1
c
w
) and the magnitude of the consequential
flux, F, is given as
F
¼
K DC
where the proportionality constant, K, has dimensions of velocity and so
is frequently referred to as the transfer velocity. The concept is elabo-
rated upon in Chapter 7.
Air-sea exchange processes are consequently dependent upon the
concentration gradient and the transfer velocity. The transfer velocity
is not a constant, but rather depends upon several physical parameters,
such as temperature, wind speed and wave state. The exchange can also
be attenuated by the presence of a surface film or slick. Alternatively, the
exchange can be facilitated by bubble formation. The concentration