Geology Reference
In-Depth Information
typical value for oceanic waters being 35 g kg
1
. In an oceanographic
context, the most important consequence of the addition of salt to water
is the effect on density. However, many of the characteristics outlined
above are also altered. The addition of electrolytes can cause a small
increase in the surface tension. This effect is not commonly observed in
seawater due to the presence of surfactants, which decrease the surface
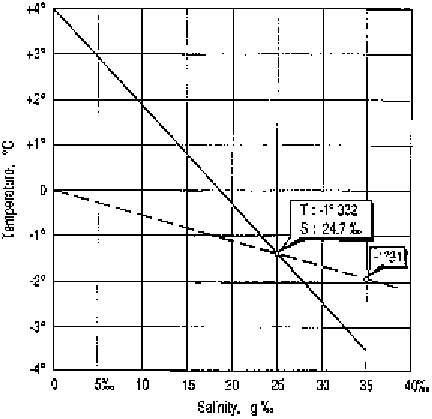
tension and so facilitate foam formation. As illustrated in Figure 2, the
presence of salt does depress the temperature of maximum density and
the freezing point of the solution relative to pure water. Thus, seawater
with a typical salt content of 35 g kg
1
freezes at approximately
1.9 1C
and the resulting ice is denser than the solution. However, more often
than the formation of sea ice itself, the freezing process tends to produce
fresh ice overlying a more concentrated brine solution. Salts can be
precipitated at much lower temperatures, i.e., mirabilite (Na
2
SO
4
.2H
2
O)
at
8.2 1C and halite (NaCl) at
23 1C. Some brine inclusions and salt
crystals can become incorporated into the ice.
From an oceanographic perspective, the fundamental properties of
seawater are temperature, salinity and pressure (i.e., depth dependent).
Together, these parameters control the density of the water, which in
turn determines the buoyancy of the water and pressure gradients. Small
density differences integrated over oceanic scales cause considerable
pressure gradients and result in currents.
Figure 2 The temperature of maximum density (—) and freezing point (- -) of seawater
as a function of dissolved salt content
(Adapted from Tchernia, 1980.
2
)