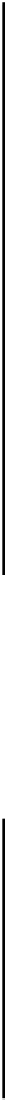Geology Reference
In-Depth Information
On the line, the concentrations of the As
III
and As
V
species are equal.
so pe
¼
log K/2 - pH
¼
9.73-pH.
Crossing points:
For the lines defined by K
1
and K
6
, substitute pH
¼
2.24 in pe
¼
9.73-
pH, so pe
¼
7.49.
20
H
3
AsO
4
O
2
/H
2
O
H
2
AsO
4
−
10
HAsO
4
2
−
H
3
AsO
3
0
H
2
O/H
2
H
2
AsO
3
−
−
10
HAsO
3
2
−
0
2
4
6
8
10
12
pH
In Examples 3.11 and 3.12 it has been assumed that no solid phases
are formed. At higher solution concentrations, however, the formation
of solid phases such as insoluble hydroxides, carbonates and sulfides
must be considered. The stability of these phases is often pe and/or pH
dependent. Redox equilibria involving both aqueous and solid phase 'S'
species are shown in Example 3.13.
Example 3.13: Construction of a pe-pH diagram for SO
4
-S
(s)
-H
2
S
system. Use S
T
¼
10
2
mol L
1
.
Acid-base behaviour of S
VI
:
HSO
4
SO
2
4
þ
H
1
K
1
¼
10
2.0
"
Acid-base behaviour of S
II
:
H
2
S
(aq)
HS
þ
H
1
K
2
¼
10
7.0
"
Reduction of S
VI
to S
0
:
HSO
4
þ
7H
1
þ
6e
K
3
¼
10
34.2
"
S
(s)
þ
4H
2
O