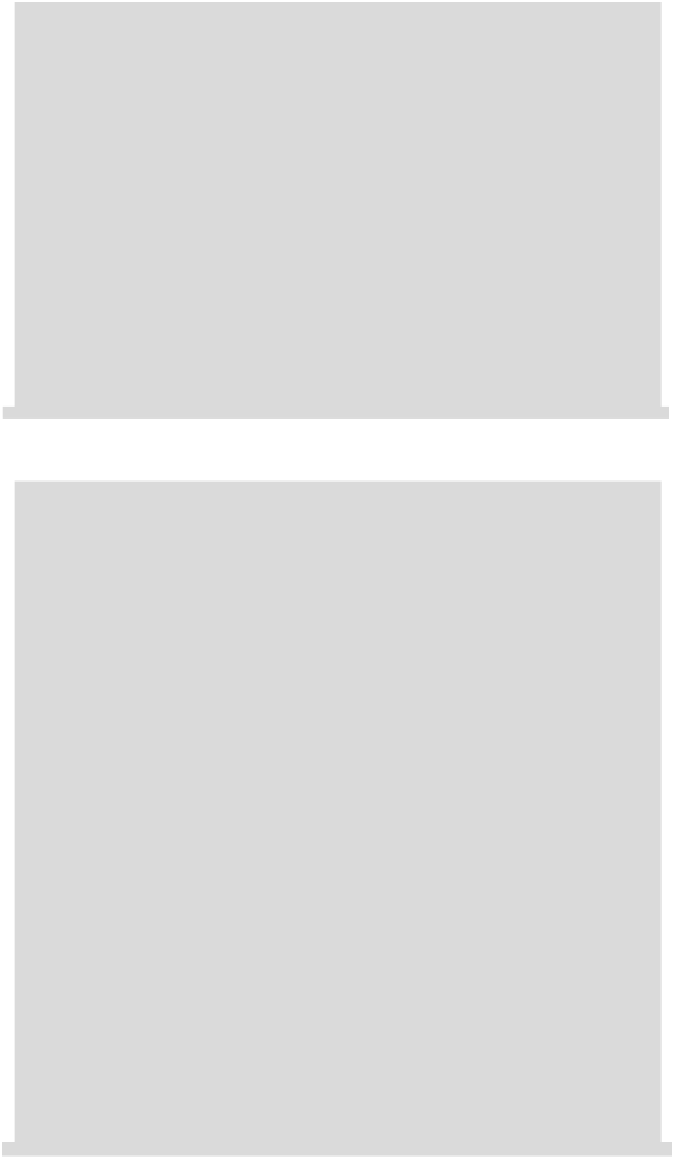
Geology Reference
In-Depth Information
30
20
O
2
/H
2
O
10
Cr
3+
CrO
4
2-
0
Cr(OH)
3
0
Cr(OH)
4
-
H
2
O/H
2
-10
0
2
4
6
8
10
12
14
pH
Example 3.12: Construction of a pe-pH diagram for As-H
2
O. Assume
that no solid phases are formed.
Acid-base behaviour of As
V
:
H
3
AsO
4
Ð
H
2
AsO
4
þ
H
þ
K
1
¼
10
2
:
24
H
2
AsO
4
Ð
HAsO
2
4
þ
H
þ
K
2
¼
10
6
:
76
HAsO
2
4
Ð
AsO
3
þ
H
þ
K
3
¼
10
11
:
60
4
Acid-base behaviour of As
III
:
H
3
AsO
3
Ð
H
2
AsO
3
þ
H
þ
K
4
¼
10
9
:
23
H
2
AsO
3
Ð
HAsO
2
3
þ
H
þ
K
5
¼
10
12
:
10
Reduction of As
V
to As
III
:
H
3
AsO
4
þ
2H
þ
þ
2e
Ð
H
3
AsO
3
þ
H
2
O
K
6
¼
10
19
:
46
H
2
AsO
4
þ
3H
þ
þ
2e
Ð
H
3
AsO
3
þ
H
2
O
K
7
¼
10
21
:
70
HAsO
2
4
þ
4H
þ
þ
2e
Ð
H
3
AsO
3
þ
H
2
O
K
8
¼
10
28
:
46
HAsO
2
þ
3H
þ
þ
2e
Ð
H
2
AsO
3
þ
H
2
O
K
9
¼
10
19
:
23
4
AsO
3
þ
4H
þ
þ
2e
Ð
H
2
AsO
3
þ
H
2
O
K
10
¼
10
30
:
83
4
AsO
3
þ
3H
þ
þ
2e
Ð
HAsO
2
K
11
¼
10
18
:
73
þ
H
2
O
4
3
Construction of vertical lines:
e.g. K
1
¼
{H
1
}{H
2
AsO
4
}/{H
3
AsO
4
}
On the line, the concentrations of the As
V
species are equal,
so pH
¼
pK
1
¼
2.24.
Construction of sloped lines:
e.g. K
6
¼
{H
3
AsO
3
}/{H
3
AsO
4
}{H
1
}
2
{e
}
2