Geology Reference
In-Depth Information
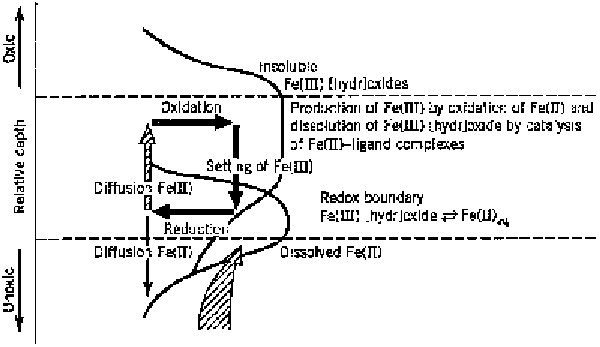
Figure 2 Oxic-anoxic boundary in the water or sediment column
15
(Reproduced with permission from ref 15,
r
John Wiley & Sons Inc. 1996)
Upper Limit
O
2(g)
þ
4e
þ
4H
1
"
2H
2
O
(1)
log K
¼
83.1
(3.76)
Where p
O2
¼
1, log p
O2
¼
0 and
log K
¼
4 log
f
e
g
4 log
f
H
þ
g
pe
¼
20
:
78
pH
ð
3
:
77
Þ
Lower Limit
2H
1
þ
2e
"
H
2(g)
log K
¼
0
(3.78)
or
2H
2
O
þ
2e
H
2(g)
þ
2OH
"
log K
¼
28
(3.79)
Where p
H2
¼
1, log p
H2
¼
0 and
log K
2log K
w
¼
2log{e
}
2log {H
1
}
or
log K
¼
2log{e
}
2log {H
1
}
In both cases:
pe
¼
pH
(3.80)
For any system, stability relationships between solution phase species
and solid phases can be used to construct pe
pH diagrams representing