Geology Reference
In-Depth Information
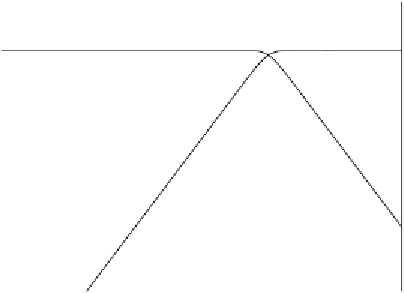
0
p
ε
= 1/n log K
{Fe
2+
}
{Fe
3+
}
5
10
15
-5
0
5
10
15
20
p
ε
Redox intensity or electron activity in natural waters is usually deter-
mined by the balance between those processes which introduce oxygen
(e.g. dissolution of atmospheric oxygen, photosynthesis) and those which
remove oxygen (e.g. microbial decomposition of organic matter). Often
these processes are controlled by the availability of inorganic nutrients
such as phosphate and nitrate, e.g. as utilized in the formation of organic
matter during photosynthesis (see Section 3.3.4).
106CO
2
þ
16NO
3
þ
HPO
2
4
þ
122H
2
O
þ
18H
1
"
(3.74)
C
106
H
263
O
110
N
16
P
1
þ
138O
2
The decay of organic matter produced in this manner leads to the
subsequent consumption of oxygen, e.g. by respiration.
106CO
2
þ
16NO
3
þ
HPO
2
4
þ
122H
2
O
þ
18H
1
(3.75)
The decay of organic matter requires the presence of a terminal electron
acceptor and in Equation (3.75) molecular oxygen is reduced to water.
Other terminal electron acceptors present in natural waters include
NO
3
,Mn
IV
,Fe
III
,SO
4
2
, and CO
2
. Once all molecular oxygen has
been consumed, organic matter is decomposed via reactions involving
other terminal electron acceptors in a series determined by the pe
intensity as shown in Table 3.
The sequence of redox reactions involving organic matter, all of which
are microbially mediated, can be thought of as progressing through
C
106
H
263
O
110
N
16
P
1
þ
138O
2
"