Geology Reference
In-Depth Information
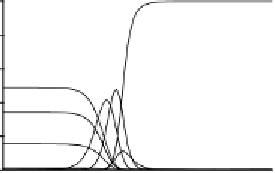
hydrolysis only
hydrolysis + fluoride + sulfate
1.0
1.0
Al(OH)
-
Al(OH)
-
Al
3+
0.8
0.8
0.6
0.6
Al
3+
AlOH
2+
Al(OH)
+
AlOH
2+
Al(OH)
+
0.4
0.4
AlF
2+
0.2
AlSO
4
+
0.2
Al(OH)
0
Al(OH)
0
AlF
2
+
0.0
0.0
02468 0 2 4
02468 0 2 4
pH
pH
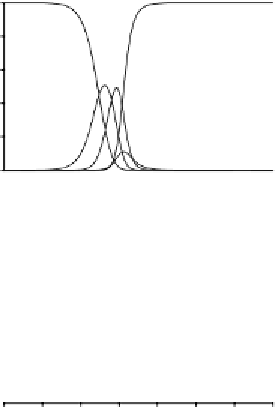
hydrolysis + fluoride
hydrolysis + fluoride + sulfate+ organic ligand
1.0
1.0
Al(OH)
-
AlOrg
2+
Al(OH)
-
0.8
0.8
Al
3+
0.6
0.6
AlOH
2+
Al(OH)
+
Al
3+
AlF
2+
0.4
0.4
AlF
2+
Al(OH)
0
0.2
0.2
AlSO
+
AlOH
2+
, Al (OH)
+
,
Al(O H)
3
0
AlF
+
AlF
2
+
0.0
0.0
02468 0 2 4
02468 0 2 4
pH
pH
Influence of Ionic Strength. It should be noted that corrections to take
account of ionic strength as discussed in Section 3.2.1.2 apply not only
to the acid-base equilibrium constants but also to the stability constants
for complex formation.
3.2.4.2 pe as a Master Variable. Chemical speciation is also influ-
enced by the redox conditions prevailing in natural waters. Although
redox reactions are often slow, and therefore species are present at
activities far from equilibrium, they are commonly represented by
thermodynamic equilibrium expressions, which can provide the bound-
ary conditions towards which a system is proceeding.
pe, a parameter describing redox intensity, gives the hypothetical electron
activity at equilibrium. It measures the relative tendency of a solution to
accept or donate electrons with a high pe being indicative of a tendency for
oxidation, i.e. accepting electrons, while a low pe is indicative of a tendency
for reduction, i.e. donating electrons. It is defined as
pe
¼
log{e
}
(3.66)
The pe scale is thus analogous to the pH scale (pH
¼
log{H
1
}), since a
low value for pe is obtained where the hypothetical {e
} is large (pH is
low where {H
1
} is large) and conversely a high value of pe is obtained