Geology Reference
In-Depth Information
Clearly, {H
2
CO
3
*} is now a constant for a fixed partial pressure of
CO
2
and is independent of pH, i.e. {H
2
CO
3
*}
¼
K
H
p
CO2
. This means
that {H
2
CO
3
} is also constant over the entire pH range.
Combining this new expression for {H
2
CO
3
*} with (3.52)-(3.53) gives
expressions for {HCO
3
} and {CO
3
2
}:
{HCO
3
}
¼
K
1
K
H
p
CO2
/{H
1
} and {CO
2
3
}
¼
K
1
K
2
K
H
p
CO2
/{H
1
}
2
The analytical activity, C, is not a constant but can be expressed as
C
¼
K
H
p
CO2
/a
0
which increases with increasing pH according to the function (1
þ
K
1
/
{H
1
}
þ
K
1
K
2
/{H
1
}
2
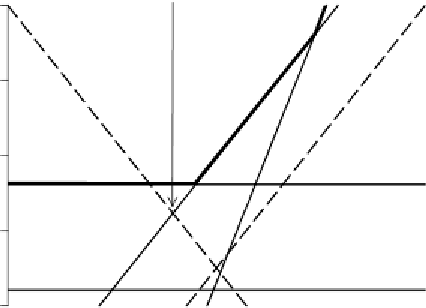
) as shown by the heavy black line on the
accompanying graph.
Plotting
log{}vs. pH for {HCO
3
} and {CO
3
2
} gives lines of slope
þ
1 and
þ
2, respectively. The charge balance expression, which is the
same as that for the closed system, can again be used to determine the
equilibrium pH.
{H
+
} ~ {HC O
-
}
0
2
4
H
2
CO
3
*
6
H
2
CO
3
8
0
2
4
6
8
10
12
14
pH
Finally, it is useful to note that the expressions describing the pH
dependence of carbonate species concentration for both closed (Ex-
ample 3.6) and open systems can also be used in mass balance
expressions for metal complexation.
pH Dependence of Complex Formation. Other equilibria such as metal
complexation reactions can be considered as acid-base reactions and
plots of log {}against pH also provide information about the dominant
species present in solution under different geochemical conditions. Hy-
drolysis of metal cations occurs progressively with increasing pH, e.g.