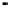Geology Reference
In-Depth Information
d log {2, 4, 6-TCP
}/d pH
¼
d log K/d pH
þ
d log C/d pH
d log{H
1
}/d pH
¼þ
1
For pH4pK,(K
þ
{H
1
})
K and
d log {2, 4, 6-TCP}/d pH
¼
d log {H
1
}/d pH
þ
d log C/d pH
d log K/d pH
¼
1
d log {2, 4, 6-TCP
}/d pH
¼
d log K/d pH
þ
d log C/d pH
d log K/d pH
¼
0
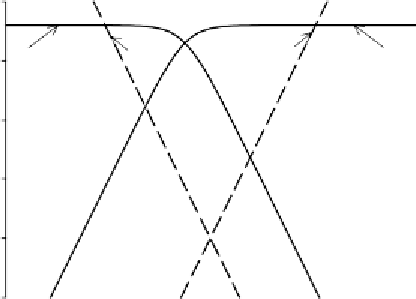
To construct the straight line sections of the graph, note that
log{2,4,6-TCP}
¼
log C for pH
o
pK and log{2,4,6-TCP
}
¼
log C
for pH4pK. Then using the full expressions for log{2,4,6-TCP} and
log{2,4,6-TCP
}, calculate several points on lines of slope
þ
1 and
1
to complete the graph.
-
3
OH
-
TCP
H
+
TCP
-
4
5
6
{H
+
} ~ {TCP
-
}
7
8
0
2
4
6
8
10
12
14
pH
The equilibrium pH for a solution containing the weak acid is
obtained by approximating the charge balance expression by
{H
1
}
{TCP
} (because the solution will be acidic and thus {OH
}
is very small). Conversely, the equilibrium pH for a solution of the
salt of the weak acid is obtained by approximating the appropriate
charge balance expression by {TCP}
B
{OH
} (because the solution
will be basic and so {H
1
} is very small).
Note also that, as shown in Example 3.4, the equilibrium constant, K,
can be corrected for ionic strength of solution. In general, the
equilibrium pH value decreases with increasing ionic strength.
B
Carbonate Equilibria. Another example of the importance of acid-base
behaviour, not only in aerated freshwaters but also in seawater (see