Geoscience Reference
In-Depth Information
100%
N
2
O
0%
100%
O
2
and O
3
0%
100%
CO
2
0%
100%
H
2
O
0%
100%
Total
atmosphere
0%
0.1
0.2
0.3
0.4
0.6
0.8
1
2
Wavelength (
1.5
3456 8 0
0 0
μ
m)
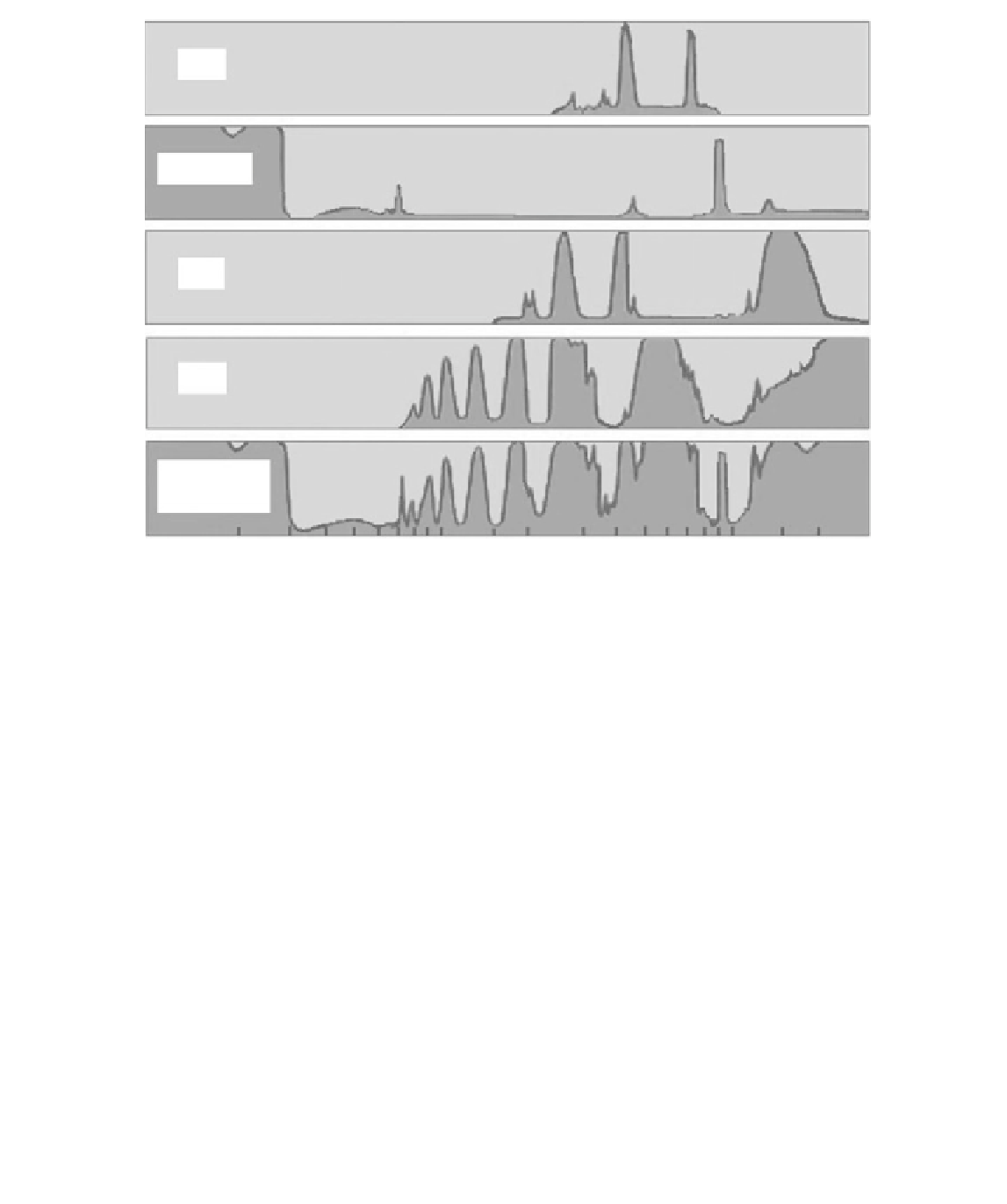
Figure 5.9
Absorption spectra of radiatively active gases in the lower atmosphere as a function of wavelength.
However, much of this radiation is re
absorbed in selected wavebands by gases in
the atmosphere, including water vapor, carbon dioxide, the oxides of nitrogen,
methane and ozone, see Fig. 5.9. This is the basis of the so
−
called
greenhouse effect
.
However, according to the Kirchoff 's principle, gases which absorb energy at a
particular wavelength also re
−
emit energy at the same wavelength and some of
this radiation is emitted downward toward the surface, so there is an associated
downward flux of radiant energy in the longwave wave band,
L
d
, which is given by:
−
4
L
= ε
s
T
(5.20)
d
atmos
atmos
At any instant, the net exchange of longwave radiation at the surface,
L
n
, is the
difference between
L
u
and
L
d
, i.e.:
LLL
=−
(5.21)
n
u
d
Figure 5.10 illustrates the spectrum of the upward and downward longwave
streams for a hypothetical case with a surface temperature of 288 K and a cloudless