Geoscience Reference
In-Depth Information
Escaping molecule
Higher saturated vapor
pressure
Lower saturated vapor
pressure
More escape and capture
Less escape and capture
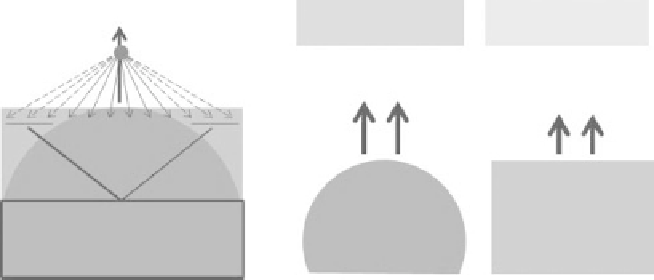
Figure 10.2
The difference
in binding force on escaping
water molecules above a
curved and flat water surface
and its consequences on
saturated vapor pressure.
Less molecular bonds
for a curved surface
although locally aerosol cloud condensation nuclei concentration can be greatly
enhanced by industrial activity and agricultural practice, including the burning of
agricultural crops and forests.
Saturated vapor pressure of curved surfaces
Chapter 2 discussed how evaporation involves two processes, the escape of
water molecules from a liquid surface when they have sufficient energy to
overcome the forces that bind them, and the capture of a proportion of the
molecules above the surface when they collide with it. These two competing
exchanges achieve equilibrium when the air above a water surface is saturated and
the vapor pressure of the air at saturation depends on the binding energy of the
molecules in the water. The lower the binding energy, the greater is the number of
molecules in the water with sufficient energy to escape and the greater is the boil
off rate. If the boil off rate is higher, the equilibrium between rates occurs when the
concentration of water vapor in the air is higher and consequently the saturated
vapor pressure is higher.
If the evaporating water surface is curved, molecules leaving the surface are on
average farther away from the molecules that remain in the water, and the effective
binding force is therefore reduced, see Fig. 10.2. Consequently, water molecules
escape more easily from a curved surface and the saturated vapor pressure of air
above a curved surface is higher as a result. The increase in saturated vapor pres-
sure above a curved surface with radius
r
relative to the saturated vapor pressure
above a flat water surface is
de
sat
, where:
r
rr
S
2
de
=
v
(10.1)
sat
−
r
w
v
where
r
v
are the densities of water and water vapor, respectively, and
S
is
the surface tension of water. Therefore, in principle, the difference in saturated
vapor pressure goes to infinity as the radius of the curved surface goes to zero.
r
w
and