Environmental Engineering Reference
In-Depth Information
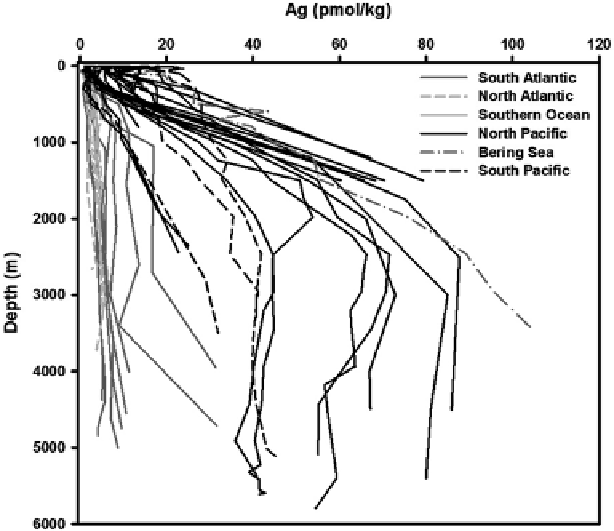
Fig. 2
Vertical profi les of silver concentrations (pmol/kg) in the oceans. Data from Murozumi
(
1981
), Martin et al. (
1983
), Flegal et al. (
1995
), Rivera-Duarte et al. (
1999
), Ndung'u et al. (
2001
),
Sañudo-Wilhelmy et al. (
2002
), Zhang et al. (
2001
,
2004
), Ranville and Flegal (
2005
), and Kramer
et al. (
2011
). Also included are previously unpublished data from our laboratory
a more effi cient transfer of silver out of surface waters in sinking particle aggregates,
and/or a relatively greater input (e.g., hydrothermal vents) of silver in deep oce-
anic waters.
In addition to silica, silver concentrations in seawater profi les also correlate with
those of copper (Fig.
4
). The position of silver immediately under copper in the
Periodic Table attests to its role as a biogeochemical analog of copper, an essential
trace element. This similarity also accounts for some of silver's toxicity to many
marine invertebrates, whose respiratory pigment is the copper-based hemocyanin
(Burmester
2002
). However, as with silica, silver concentrations deviate from a true
linear correlation with those of copper, especially at higher concentrations—again
attesting to dissimilarities in the biogeochemical cycles of the two elements.
Those differences are illustrated in Table
3
, which lists concentrations of silver,
silicate and copper that are present in hydrothermal plumes. Based on the sea water
concentrations of silver (0.023 nmol/kg) and copper (0.0033
mol/kg), as given in
the original compilation by Douville et al. (
2002
), the atomic ratio of silver to cop-
per in the plumes (3.4-8.9 × 10
−4
) is one order of magnitude lower than it is in sea
water. Consequently, the relative excess of silver compared to copper in deep ocean
water is not due to a simple enrichment from hydrothermal inputs. But differences
ʼ
Search WWH ::

Custom Search