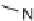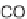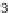Biomedical Engineering Reference
In-Depth Information
SCHEME 3.14
Intramolecular inverse electron-demand Diels-Alder reaction of tryptophan
derivative triazines.
appeared to be critical in the transition state, explaining the high
exo
selectivity when
cyclohexene, norbornene, and cyclooctene were used as dienophiles. In contrast to
electron-rich dienophiles, facial selectivity was observed with unactivated olefins,
and a single diastereomer was obtained (
47
).
Snyder's group reported a synthetic pathway in which tryptophan derivatives
were joined to triazines before an intramolecular inverse electron-demand Diels-
Alder reaction occurred (Scheme 3.14) [27]. The acylation of the inverse electron-
demand Diels-Alder precursor
48
was required to lower the lowest unoccupied
molecular orbital (LUMO) of the triazines, allowing for the cycloaddition reaction
to occur upon heating. Using this general synthetic approach, three main scaffolds
were synthesized (e.g.,
49
) and further functionalized on
N
14 with the use of sulfonyl
and acyl chlorides. 199 Indoline alkaloid scaffolds possessing high complexity and
diversity were synthesized [28]. Several hits that weakly inhibited the growth of a
number of
Plasmodium falciparum
lines were found.
Recently, Dow, Murrison, and co-workers demonstrated that when the synthe-
sis and folding of substrates took place in a single reaction, the efficiency could
increase. Accordingly, they reported the synthesis of several alkaloid-like scaffolds
using a three-component reaction of secondary amines, carbonyl compounds, and tri-
azines (Scheme 3.15) [21b,29]. Inverse electron-demand Diels-Alder cycloaddition
reactions of triazines with previously generated enamines yielded 2-azadienes
50
,
which were ideal substrates for subsequent folding reactions. When a dienophile
is present within the molecule, 2-azadienes
50
can undergo an intramolecular
Diels-Alder reaction, thus giving highly complex and diverse alkaloid-like scaffolds
(
51
to
53
).
3.3
1,3-DIPOLAR CYCLOADDITION REACTIONS
The 1,3-dipolar cycloaddition reaction is an important transformation for the con-
struction of polyheterocyclic molecules. The concurrent formation of two carbon-
carbon bonds, yielding cyclic or even bicyclic heterocycles, makes this reaction a
good choice for DOS.