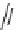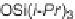Biomedical Engineering Reference
In-Depth Information
SCHEME 3.1
Pairwise use of complexity-generating reactions involving Ugi
four-
component and intramolecular Diels-Alder reactions.
adducts
6
as single regio- and diastereoisomers, which were efficiently converted to
paracyclophanes
7
upon heating. More than 4000 skeletally diverse small molecules
were synthesized through this strategy.
Aube et al. reported tandem reaction sequences in which they combined a Diels-
Alder reaction with an intramolecular Schmidt reaction [12] to access
Stemona
[13]
alkaloid-like scaffolds (Scheme 3.4) [14]. When the Diels-Alder reaction was fol-
lowed by an imide acylation reaction, octahydroisoquiol-1-one-8-carboxylic acids
were afforded (Scheme 3.5) [15]. With regard to the azido-Schmidt Diels-Alder
reaction sequence, the authors reported two ways to combine the individual reac-
tions. One strategy used the Diels-Alder cycloaddition reaction to join together the
azide and ketone present on the diene and dienophile, respectively, followed by the
intramolecular Schmidt reaction (Scheme 3.4a). In the second strategy, the azide
and ketone, the latter in the form of enone, were both present on the dienophile,
and the Schmidt reaction occurred only after elimination of the conjugation during
the Diels-Alder reaction (Scheme 3.4b). Both strategies, catalyzed by Lewis acids,
proceeded through an endo-selective Diels-Alder cycloaddition followed by stere-
oselective Schmidt ring expansion, generating polycyclic compounds (
8
and
9
) with
good yields and high diastereoselectivity.