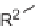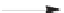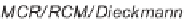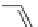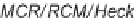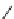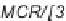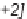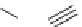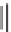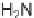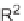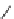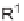Biomedical Engineering Reference
In-Depth Information
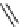
SCHEME 2.24
Sequential MCR/cyclization strategy for DOS reported by Sunderhaus
et al. [68].
can be exploited for further diversification, taking advantage of the pair phase of
the B/C/P strategy. In a 2007 report by Sunderhaus et al. sequential reactions of
imines, acid chlorides, and nucleophiles were developed to prepare multifunctional
substrates that were employed in subsequent ring-forming reactions [68]. These sub-
sequent reactions included RCM, Dieckmann and Heck reactions, and Diels-Alder
and dipolar cycloadditions to assemble a varied collection of functionalized hetero-
cyclic scaffolds (Scheme 2.24).
The biological importance of indoles has led to a large number of strategies to
form the indole ring system [69,70]. One branch of these strategies is the employment
of transition metal catalysts to mediate formation of the indole ring system; indeed,
multicomponent variants of these reactions have emerged. Kaspar and Ackermann
have developed a three-component reaction starting with
o
-dihaloarenes involving a
Sonagashira coupling followed by Buchwald-Hartwig coupling and ending with an
intramolecular hydroamination (Scheme 2.25) [71].
In a related reaction, Knapp and Kurth utilized
o
-dihaloarenes with amines and
aldehydes or ketones in a three-component reaction [72]. This transformation first
SCHEME 2.25
3CR Sonagashira/Buchwald-Hartwig/hydroamination indole synthesis.