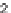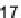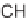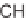Biomedical Engineering Reference
In-Depth Information
SCHEME 2.21
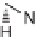
MCAP reported by Martin et al. [61] as the key step in the total synthesis
of the indole alkaloid tetrahydroalstonine and as a means of generating a diverse library of
compounds based on the natural product scaffold.
both target- and diversity-oriented synthesis [62,63]. Recently, an MCAP was used
as the key step in the total synthesis of the indole alkaloid tetrahydroalstonine and
has also been used to generate a diverse library of compounds based on the natural
product scaffold (Scheme 2.21) [64].
Another MCAP library synthesis recently reported by Martin's group utilizes a
reductive amination and azide-alkyne dipolar (Huisgen) cycloaddition sequence to
access a 1,2,3-triazolo-1,4-benzodiazepine core [65]. The MCR products were further
diversified using N-functionalization and
-aminonitrile chemistry. Compounds con-
taining the 1,4-benzodiazepine ring system bind to many different targets, including
G protein-coupled receptors (GPCRs), ligand-gated ion channels, and enzymes [66].
This ring system is also a common structural subunit in numerous pharmaceutical
agents, biological probes, and bioactive natural products. The use of a MCAP in this
case led to the rapid assembly of a library of over 100 compounds for high-throughput
screening (Scheme 2.22).
Although the term
MCAP
has only been applied to research reported by Martin's
group, other efforts in diversity-oriented synthesis follow a very similar strategy.
For instance, Shaw's group has recently reported a formal cycloaddition between
cyanosuccinic anhydride and imines to form
-lactams with multiple stereocenters