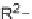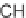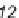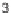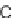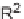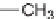Biomedical Engineering Reference
In-Depth Information
Σ
SCHEME 2.7
4CR reported by Wei and Shaw [31], leading to the formation of
-lactams.
SCHEME 2.8
4CR catalyzed by L-proline for the formation of pyridocarbazoles.
is highly variable in this particular reaction, all the carbazole products were prepared
in high yield (Scheme 2.8), and a structure-activity relationship was established for
the antimicrobial, antioxidant, and cytotoxic properties of this scaffold.
Knapp and Kurth have also developed a one-pot 2
3CR that effectively assem-
bles in one pot isoxazolines from four components (Scheme 2.9) [34]. Here a two-
component reaction between an acyl ketene and amine forms a
·
-ketoamide. In this
SCHEME 2.9
2
·
3 Component synthesis of
-ketoamide isoxazolines.