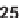Biomedical Engineering Reference
In-Depth Information
SCHEME 2.3
Formation of spirobenzofuranones via an isocyanide-based 3CR, a scaffold
seen in the antifungal drug griseofulvin (
28
).
One of the more interesting electrophiles seen in MCRs with isonitriles are arynes,
which, when allowed to react, generate a highly reactive 1,3-zwitterionic intermediate
that can lead to a wide variety of structures, depending on the third component present
in the reaction [26]. Stoltz's group discovered a novel 3CR of arynes, isocyanides,
and phenyl esters to provide phenoxy-iminoisobenzofurans in good yield [27]. The
arynes were generated in situ from 2-(trimethylsilyl)aryl triflates [28] using a fluoride
source, and the formation of the unusual heterocyclic scaffold took place under mild
conditions. Interestingly, when the ester component of this MCR was replaced by
an electron-deficient alkyne (internal or terminal), the reaction provided carbocyclic
iminoindenones in good yield. Worthy of note is the selective addition of isocyanides
to arynes in the presence of electrophilic alkynes (Scheme 2.5).
Another interesting example of an MCR involving arynes and isocyanides was
reported by Sha and Huang [29], wherein polysubstituted pyridines and isoquinolines
were obtained with excellent selectivity using terminal alkynes as the third component
(Scheme 2.6). The selectivity observed between these two heterocycles was dependent
on the reaction conditions, as pyridines were formed with an excess of terminal
alkynes, and isoquinolines were formed with an excess of arynes. This MCR serves as
a mild preparation of these heterocycles, which often involve harsh reaction conditions
or complicated procedures [30].
SCHEME 2.4
Interrupted Ugi-acylation reaction.