Biomedical Engineering Reference
In-Depth Information
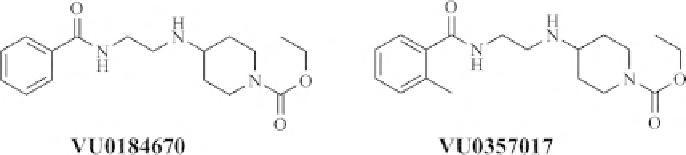
FIGURE 18.4
M
1
acetylcholine receptor agonists.
target an allosteric site on the receptor [45]. It is known that the M
1
,M
3
, and
M
5
receptors function through the phosphoinositol system and the phospholipase
C effector to stimulate intracellular calcium release. Based on this function, a cell-
based fluorescence assay using the calcium-binding dye Fluo-4 was developed for
screening 65,000 compounds in the Molecular Libraries Production Center Network
(MLPCN) library to identify compounds that increase intracellular calcium levels in
a CHO cell line expressing the M
1
receptor [74]. Optimization of the initial hits from
the screen produced compounds VU0184670 and VU0357017 as highly subtype-
selective agonists of the M
1
receptor (Figure 18.4), even at the highest compound
concentrations tested (30
M). Moreover, the compounds exhibited no significant
activity in a screen of 68 GPCRs, ion channels, and transporter targets.
Site-directed mutagenesis experiments were employed to characterize the binding
properties of the agonists identified. A mutation that reduced the affinity of acetyl-
choline and other orthosteric ligands of the receptor had little effect on VU0184670
and VU0357017 agonism [75]. The orthosteric M
1
antagonist atropine blocked the
response to VU0184670 in a noncompetitive fashion, further suggesting that binding
occurs at an allosteric site. Mutations in the third extracellular loop (ECL3) of the
M
1
receptor reduced VU0184670-induced activation, suggesting that this loop may
harbor the allosteric binding site.
The compounds were further characterized in a variety of cellular and in vivo
experiments. VU0184670 was shown to potentiate the
N
-methyl-D-aspartate gluta-
mate receptor (NMDAR) current in whole-cell patch clamp experiments with hip-
pocampal CA1 pyramidal cells, an effect that has been postulated to mediate cholin-
ergic function in Alzheimer's disease and schizophrenia [76]. In murine models, both
compounds were found to penetrate the CNS after intraperitoneal administration,
with VU035017 being cleared more readily over time.
In a rodent model, VU035017 was shown to reverse the cognitive deficits induced
by the muscarinic receptor antagonist scopolamine. Rats were conditioned to exhibit
freezing behavior upon exposure to a neutral conditioned stimulus by undergoing a
training period in which the neutral conditioned stimulus was coupled with a mild
footshock. Scopolamine interfered with the acquisition of this response. However,
VU035017 administered intraperitoneally at 10 mg/kg reversed the scopolamine-
induced effects, with animals receiving both compounds acquiring the contextual
fear conditioning response.