Biomedical Engineering Reference
In-Depth Information
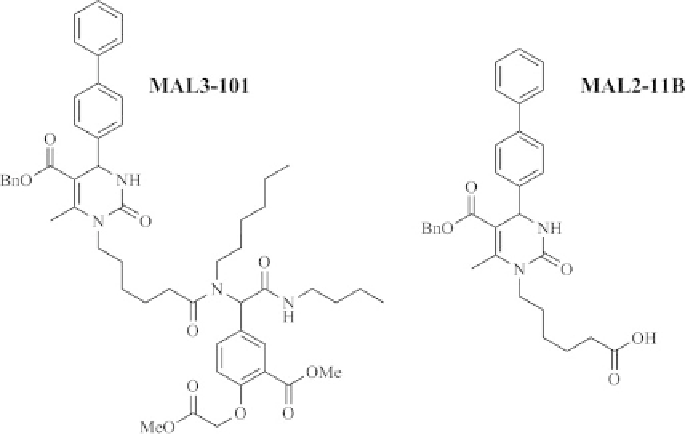
FIGURE 18.3
Inhibitors of Hsp70 ATPase activity.
hydrolyzed over the time course was used to approximate the first-order rate constant
of hydrolysis. This assay was used to measure both endogenous Hsp70 ATPase
activity and J-domain-stimulated Hsp70 ATPase activity. To test the latter activity,
the yeast Hsp40Ydj1p protein or the SV40 TAgwere premixedwith the Hsp70 Ssa1p-
[
-
32
P]ATP complex prior to addition of the compound. Several compounds in the
library were identified that either enhanced or decreased endogenous or J-domain-
stimulated Hsp70 ATPase activity. Most interestingly, the compound MAL3-101 was
found to specifically reduce TAg-stimulated ATPase activity in a dose-dependent
manner while exhibiting little impact on endogenous or Ydj1p-stimulated ATPase
activity (Figure 18.3). The compound was further shown to inhibit the Hsp40/Hsp70-
dependent translocation of a protein, yeast pp
F, into yeast endoplasmic reticulum
(ER)-derived microsomes.
MAL3-101 proved to be useful as a probe for studying a variety of chaperone-
related processes. In one study, MAL3-101 was used to examine the mode by which
eight different tail-anchored (TA) proteins integrate into a mammalian ER membrane
[67]. Integration of TA proteins into the ER membrane is promoted by two separate
complexes: the Hsp40/Hsc70 chaperone complex (Hsc70, a member of the Hsp70
family of proteins), and the Asna-1 ATPase complex [68]. In this study, MAL3-
101 was found to significantly inhibit ER integration of three TA proteins (human
Bcl2, PTP1B, and Cb5), suggesting that functional Hsp40/Hsc70 is necessary for their
insertion; however, the integration of five other TA proteins (rat Syb2 and Syn1A; and
human Ubc6J1, Sec61b, and Ramp4) was not affected by the compound, suggesting
that a different pathway, probably involving the Asna-1 ATPase complex, is sufficient
for insertion of these proteins.