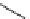Biomedical Engineering Reference
In-Depth Information
F
Me
HO
N
S
OO
2
5
Me
N
O
6
N
N
Me
O
Me
O
O
12
HTS Hit Compound
31
Stereochemistry (
C
2
C
5
C
6
C
12
)
Stereochemistry: SRRS
Dd2 (EC
50
): 120 nM
3d7 (EC
50
): 120 nM
Hemolysis (EC
50
): >40
μ
M
Solubility (water): <500 nM
RSSR
SRRS
RSRR
SRSS
SSSS
RRRR
SSSR
RRRS
RSSS
SRRR
SSRS
RRSR
SSRR
RRSS
RSRS
SRSR
0
50
100
% inhibition at 280 nM
FIGURE 17.12
SSAR of hit compound
31
.
As was the case with NITD609, biological activity was dependent on the com-
pound's stereochemistry. SSAR analysis of all 16 isomers of macrocycle
30
showed
that biological activity is observed in just two isomers having the same ring config-
uration and differing only in the exocyclic C2 stereochemistry (Figure 17.12). The
most potent compound,
SRRS-31
, was not toxic to erythrocytes but was insoluble
in aqueous solution (
M in water), which hindered analysis in further assays.
SAR studies were initiated on this compound focusing on substituents at three areas
of the molecule: (1) the aniline site, (2) the amine side chain, and (3) the lactam side
chain, with a goal of improving potency and aqueous solubility.
The synthesis of analogs was performed in solution and followed the strategy
reported by Marcaurelle et al. [16c]. In general, the analogs were prepared through
an initial acylation of aromatic acid
28
with linear amine
6
, which was derived from
an anti-selective aldol reaction adapting the ephedrine-based protocols reported by
Inoue, Abiko, and co-workers [21]. The resulting amides were subjected to TBS
deprotection and allylation to give RCM precursors
29
in three steps. The outcome of
the RCM macrocyclization was found to be highly dependent on the stereochemistry
at C5 and C6, with those derived from the anti-selective asymmetric aldol reaction
being much more efficient and facile then those derived from syn-selective aldol reac-
tion. Thus, compound
29
readily provided the 14-membered macrocycle as a mixture
of
E
/
Z
isomers using the second-generation Hoveyda-Grubbs catalyst (Scheme 17.5).
Reduction of a mixture of olefins and a nitro group provided the first opportunity for
<
0.5